Bioengineering: Big Potential in Tiny 3D Heart Chambers
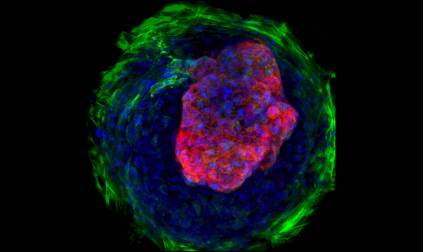
Caption: Heart microchamber generated from human iPS cells; cardiomyocytes (red), myofibroblasts (green), cell nuclei (blue)
Credit: Zhen Ma, University of California, Berkeley
Credit: Zhen Ma, University of California, Berkeley
The adult human heart is about the size of a large fist, divided into four chambers that beat in precise harmony about 100,000 times a day to circulate blood throughout the body. That’s a very dynamic system, and also a very challenging one to study in real-time in the lab. Understanding how the heart forms within developing human embryos is another formidable challenge. So, you can see why researchers are excited by the creation of tiny, 3D heart chambers with the ability to exist (see image above) and even beat (see video below) in a lab dish, or as scientists say “in vitro.”
To achieve this feat, an NIH-funded team from University of California, Berkeley, and Gladstone Institute of Cardiovascular Disease, San Francisco turned to human induced pluripotent stem (iPS) cell technology. The resulting heart chambers may be miniscule—measuring no more than a couple of hair-widths across—but they hold huge potential for everything from improving understanding of cardiac development to speeding drug toxicity screening.
Let me remind you that iPS cells are derived from genetically reprogrammed skin cells or white blood cells and have the potential to develop into many different types of cells. Scientists have had the recipe for producing cardiac cells from iPS cells for some time. But, as researchers led by Zhen Ma, Kevin Healy, and Bruce Conklin show in the journal Nature Communications [1], it turns out that encouraging those cells to undergo a more complex process akin to early heart development requires not only the right chemistry, but also the right geometry.
To provide such cues, the researchers placed undifferentiated iPS cells on specially engineered tissue culture dishes etched with tiny circles, which kept the cells tightly packed together. After two weeks confined to such close quarters, the cells that had started growing into a flat sheet of cardiac tissue spontaneously assumed a ball-like, 3D structure. The newly formed cardiac cells had organized themselves into tiny, pulsating microchambers, reminiscent of structures that form during early development of a human heart—pretty amazing stuff!
The researchers exposed all of the cells on those lab dishes to the same list of chemical ingredients in the same concentrations. It was their position on those lab dishes relative to one another that led them to become different types of cardiac cells. Cells along the edge of each of those tiny circles, which experienced greater mechanical stress and tension, appeared more like tough connective tissue—reminiscent of the pericardium that surrounds a human heart. In contrast, cells in the middle developed into cardiac muscle cells. At the center of this 3D ball of cells was a hollow space, similar to the chambers found in a living, beating heart.
Continued study of these microchambers promises to expand understanding of normal and abnormal development of the human heart. That’s welcome news considering that heart defects are the most common of all birth defects, affecting more than 35,000 babies in the United States every year [2]. And, for obvious reasons, most of what we know about heart development so far has come from studies in laboratory animals.
These microchambers may also be put to work in “organs-on-a-chip” to screen drugs or candidate drugs rapidly for toxic effects that could interfere with the development of a baby’s heart in the womb. As a demonstration of this potential use, the researchers studied the effect of the drug thalidomide on developing microchambers. Thalidomide is notorious for its tragic history of causing serious birth defects, including those affecting the heart, when taken by pregnant women. Indeed, human microchambers exposed to thalidomide in a lab dish failed to develop properly. Not only were the chambers stunted, they beat more slowly than microchambers not exposed to thalidomide.
This advance comes as a reminder of the gains to be realized in medicine when basic cell biology meets bioengineering. In fact, the work represents just one of many NIH-funded efforts, including those supported by the National Center for Advancing Translational Science’s Tissue Chip for Drug Screening Program, to produce bioengineered devices to better predict drug toxicity. While this study is focused on the heart, the same principles could be applied to the study of the liver, kidney, lung, pancreas, and many other organs.
References:
[1] Self-organizing human cardiac microchambers mediated by geometric confinement. Ma Z, Wang J, Loskill P, Huebsch N, Koo S, Svedlund FL, Marks NC, Hua EW, Grigoropoulos CP, Conklin BR, Healy KE. Nat Commun 14 July 2015 [Epub ahead of print]
[2] What are congenital heart defects? National Heart, Lung, and Blood Institute (NHLBI). July 1, 2011.
Links:
Tissue Chip for Drug Screening Program (National Center for Advancing Translational Sciences/NIH)
Stem Cell Basics (NIH)
Conklin Lab (Gladstone Institutes/University of California, San Francisco)
Healy Lab (University of California, Berkeley)
NIH Support: National Center for Advancing Translational Sciences; National Heart, Lung, and Blood Institute; National Institute of Biomedical Imaging and Bioengineering


































No hay comentarios:
Publicar un comentario