

FDA takes additional action to better understand safety of Essure, inform patients of potential risks
FDA has taken actions to provide important information about the risks of using Essure and to help women and their doctors be better informed of the potential complications associated with implantable forms of sterilization. FDA issued a new, mandatory clinical study for Essure to determine heightened risks for particular women. FDA also intends to require changes to product labeling, including a boxed warning and a Patient Decision Checklist to help to ensure women receive and understand information regarding the benefits and risks of this type of device. FDA has issued a draft guidance to provide the public an opportunity to comment on the proposed language to be included in these warnings. Since Essure’s approval in 2002, the agency has continued to monitor Essure’s safety and effectiveness by reviewing the medical literature, clinical trial information, post-approval study data and medical device reports submitted to the agency. The new actions announced today take additional steps and follow the agency’s careful evaluation of available information. More information |
Comunicaciones de la FDA sobre la seguridad de los medicamentos en español
Descargo de responsabilidad: La FDA reconoce la necesidad de proporcionar información importante sobre seguridad de los medicamentos en idiomas distintos al inglés. Hacemos lo mejor posible para proporcionar versiones en español precisas y oportunas de nuestras Comunicaciones de Seguridad de Medicamentos. Sin embargo, en caso que existiera discrepancias entre las versiones en inglés y la de español, la información contenida en la versión en inglés es la que se considera como versión oficial. Si tiene alguna pregunta, por favor contáctese con Division of Drug Information en druginfo@fda.hhs.gov.Comunicaciones de la FDA |


FDA recognizes the significant public health consequences that can result from drug shortages and takes tremendous efforts within its legal authority to address and prevent drug shortages. These shortages occur for many reasons, including manufacturing and quality problems, delays, and discontinuations. When issues are discovered by the company or the public and reported to FDA or are found by FDA upon inspection, FDA works closely with the firm to address risks involved to prevent harm to patients. FDA also considers the impact a shortage would have on patient care and access and works with the firm to restore supplies while also ensuring safety for patients. More information
|
Drug Shortages Voluntarily Reported by Manufacturers During the Past 2 Weeks:
Drug Shortages Reported to be Resolved by Manufacturers During the Past 2 Weeks:
- Aprepitant (Emend) Capsules
- Ketorolac Tromethamine Injection
- Methylphenidate Hydrochloride ER Capsules/Tablets
- Nebivolol (BYSTOLIC) Tablets
Drugs Reported to be Discontinued by Manufacturers During the Past 2 Weeks:
La FDA reconoce las consecuencias significativas para la salud pública que pueden resultar de la escasez de medicamentos y hace un gran esfuerzo dentro de sus facultades legales para abordar yprevenir la escasez de medicamentos. La escasez se produce por muchas razones, incluyendoproblemas de fabricación y calidad, retrasos y discontinuación del producto. Cuando los problemas son descubiertos por la empresa o el público y reportados a la FDA o se descubren por inspecciones de la FDA, la FDA trabaja en estrecha colaboración con la empresa para hacer frente a los riesgosinvolucrados y evitar daños a los pacientes. La FDA también considera el impacto que una escaseztendría en la atención médica del paciente y al acceso del producto y trabaja con la empresa pararestablecer el suministro al tiempo que garantiza la seguridad de los pacientes. Más información
|
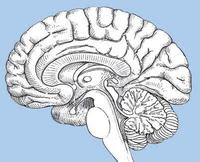
FDA approves Briviact to treat partial onset seizures
FDA has approved Briviact (brivaracetam) as an add-on treatment to other medications to treat partial onset seizures in patients age 16 years and older with epilepsy. Epilepsy is a brain disorder that causes people to have recurring seizures. A seizure is an episode, usually of relatively short duration, of abnormal brain activity. Seizures can cause a variety of symptoms, including uncontrolled movements or spasms, abnormal thinking and behavior, and abnormal sensations. Muscle spasms can be violent, and loss of consciousness can occur. Seizures occur when clusters of nerve cells (neurons) in the brain undergo uncontrolled activation. A partial onset seizure begins in a limited area of the brain. More information
FDA approved everolimus (Afinitor, Novartis) for the treatment of adult patients with progressive, well-differentiated non-functional, neuroendocrine tumors (NET) of gastrointestinal (GI) or lung origin with unresectable, locally advanced or metastatic disease
This approval was based on demonstration of improvement in progression-free survival (PFS) in a multicenter, randomized (2:1), placebo-controlled trial of everolimus 10 mg orally once daily plus best supportive care (BSC) to placebo plus BSC. The clinical trial enrolled 302 patients with unresectable, locally advanced or metastatic, well differentiated (low or intermediate grade), non-functional (no current or prior history of carcinoid symptoms), neuroendocrine tumors (NET) of gastrointestinal or lung origin. Median PFS were 11 months and 3.9 months in the everolimus and placebo arms, respectively. At the planned interim analysis, there was no statistically significant difference in overall survival between arms. More information
FDA approved obinutuzumab (Gazyva Injection, Genentech, Inc.) for use in combination with bendamustine followed by obinutuzumab monotherapy for the treatment of patients with follicular lymphoma (FL) who relapsed after, or are refractory to, a rituximab-containing regimen
This approval was based on demonstration of an improvement in progression-free survival (PFS) in a randomized, open-label, multicenter trial in patients with FL who had no response to or have progressed during or within 6 months of a rituximab-containing regimen. This trial compared 6 cycles of obinutuzumab plus bendamustine combination therapy followed by continued obinutuzumab monotherapy for up to 2 years with 6 cycles of bendamustine therapy. Efficacy was assessed in 321 patients with follicular lymphoma randomized to either obinutuzumab plus bendamustine (n=155) or bendamustine (n=166). The median age was 63 years (range 34-87). Patients had received a median of 2 prior therapies (range 1-10). The independent review assessed median PFS was 13.8 months in the bendamustine arm while the median PFS was not reached in the obinutuzumab plus bendamustine arm [HR 0.48 (95% CI: 0.34-0.68), log-rank test p-value < 0.0001). More information
FDA approved palbociclib (IBRANCE Capsules, Pfizer, Inc.) in combination with fulvestrant for the treatment of women with hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative advanced or metastatic breast cancer with disease progression following endocrine therapy
This approval is based on the demonstration of an improvement in progression - free survival (PFS) in an international, randomized, double-blind, parallel group, multicenter study comparing palbociclib plus fulvestrant to placebo plus fulvestrant. Women enrolled had HR-positive, HER2-negative advanced or metastatic breast cancer with disease progression on or after prior adjuvant or metastatic endocrine therapy. The major efficacy outcome measure was investigator-assessed PFS evaluated according to RECIST V1.1. The study demonstrated an improvement in PFS with a hazard ratio of 0.46 (95% CI: 0.36, 0.59; p< 0.0001). The median PFS was 9.5 versus 4.6 months for patients treated in the palbociclib plus fulvestrant and placebo plus fulvestrant arms, respectively. More information |
For information on drug approvals or to view prescribing information and patient information, please visit Drugs@FDA or DailyMed.

View FDA's Comments on Current Draft Guidance page, for a list of current draft guidances and other topics of interest for patients and caregivers.
|

FDA Statement on Senate Confirmation of Dr. Robert M. Califf, by Stephen Ostroff"Today the U.S. Senate voted in support of the confirmation of Dr. Robert Califf, M.D. to be Commissioner of U.S. Food and Drug Administration. Dr. Califf has demonstrated a long and deep commitment to advancing the public health throughout his distinguished career as a physician, researcher, and leader in the fields of science and medicine. He understands well the critical role that the FDA plays in responding to the changes in our society while protecting and promoting the health of the public, across the many areas we regulate – and I am confident that our public health and scientific contributions will further grow under his exceptional leadership."
Federal judge approves consent decree with Florida dietary supplement distributor, ViruxoA Florida dietary supplement distributor has been ordered by a federal court to stop selling its product, which it claimed could treat herpes. The complaint, filed by the U.S. Department of Justice on behalf of the U.S. Food and Drug Administration, sought a permanent injunction against Hill for unlawfully distributing an unapproved new drug and misbranded drug. The complaint also includes a civil fraud charge for Hill’s intent to defraud consumers by promoting his product to cure, mitigate, treat, or prevent a disease despite the absence of well-controlled clinical studies or any other credible scientific evidence to substantiate his claims. More information
FDA providing $2 million in new grants for natural history studies in rare diseases
FDA has announced the availability of $2 million in research grants to fund natural history studies in rare diseases. The aim is to collect data on how specific rare diseases progress in individuals over time so that knowledge can inform and support product development and approval. This will be the first time the FDA will provide funding through its Orphan Products Grants to conduct these types of studies for rare diseases. Natural history is the course a disease takes in affected individualsfrom the time immediately prior to its inception, progressing through a presymptomatic phase and different clinical stages, to a final outcome in the absence of treatment. This type of information is often not available, or incomplete, for many rare diseases. More information |

Have a Problem? Contact the New Ombudsman in the Office of Regulatory Affairs, by Melinda K. Plaisier is FDA’s Associate Commissioner of Regulatory AffairsWhether we are inspecting your facilities, sampling your products, or conducting investigations, the primary goal of FDA’s Office of Regulatory Affairs (ORA) is to protect the public. But I understand the impact our actions can have on you, so I am committed to making ORA’s processes as transparent as possible and quickly addressing problems you may encounter. That’s why I’m happy to announce a new resource: an ORA ombudsman who can help you with unresolved concerns. While you may continue to bring issues to my staff, Ombudsman Jessica Zeller is dedicated solely to helping you with assessing and resolving problems. Jessica, who has worked in both industry and government, understands that FDA’s perspective is often different from that of industry and other stakeholders.
To read the rest of this post, see FDA Voice Blog, March 1, 2016.
FDA issues recommendations to reduce the risk of Zika virus transmission by human cell and tissue products
As an additional safety measure against the emerging Zika virus outbreak, FDA has issued new guidance for immediate implementation providing recommendations to reduce the potential transmission risk of Zika virus from human cells, tissues, and cellular and tissue-based products (HCT/Ps). The guidance addresses donation of HCT/Ps from both living and deceased donors, including donors of umbilical cord blood, placenta, or other gestational tissues. The new guidance is a part of the FDA’s ongoing efforts to protect HCT/Ps and blood products from Zika virus transmission. On Feb. 16, the FDA issued recommendations for reducing the risk of Zika virus via blood transfusion in the U.S. More information |

FDA advisory committee meetings are free and open to the public. No prior registration is required to attend. Interested persons may present data, information, or views, orally at the meeting, or in writing, on issues pending before the committee.
Other types of meetings listed may require prior registration and fees.
Public Workshop - Advancing the Development of Biomarkers in Traumatic Brain Injury
Date: March 3, 2016 This workshop aims to examine potential biomarkers, discuss the challenges and solutions related to biomarker development methodologies, and establish strategies for data standardization, sharing and analysis of big data sets for Traumatic Brain Injury (TBI). By convening the relevant stakeholders, the goal is to obtain input on the scientific, clinical, patient and regulatory considerations associated with TBI biomarker development to improve diagnosis and clinical utility for TBI. More information
View FDA's Calendar of Public Meetings page for a complete list of meetings and workshops.
|
Please visit FDA’s Advisory Committee page to obtain advisory committee meeting agendas, briefing materials, and meeting rosters prior to the meetings. You may also visit this page after meetings to obtain transcripts, presentations, and voting results. For additional information on other agency meetings please visit Meetings, Conferences, & Workshops.
|

Hydrogen Peroxide Solution for Cleaning and Disinfecting Contact Lenses
Hydrogen peroxide and multipurpose solutions both clean and disinfect contact lenses by breaking up and removing trapped debris, protein, and fatty deposits (lipids). Unlike multipurpose solutions, hydrogen peroxide solutions are preservative-free, which makes them a suitable option for those who are allergic or sensitive to the preservatives found in multipurpose solutions. They are not risk-free, however, and should be used with appropriate cautions.More information |
More Consumer Updates
For previously published Consumer Update articles that are timely and easy-to-read and cover all FDA activities and regulated products. More information
For previously published Consumer Update articles that are timely and easy-to-read and cover all FDA activities and regulated products. More information
En Español
La información en esta página es para el público en general, y para profesionales y educadores de salud. Esta información puede ser distribuida y publicada sin previa autorización. En Español
La información en esta página es para el público en general, y para profesionales y educadores de salud. Esta información puede ser distribuida y publicada sin previa autorización. En Español
FDA Posts Questions and Answers on Spice Safety
FDA has posted Questions & Answers on Improving the Safety of Spices. The Q&A addresses spice sampling conducted at the retail level, which is still being analyzed, in addition to sampling FDA has previously conducted at import. The Q&A also addresses how the FDA Food Safety Modernization Act rules are expected to improve spice safety and discusses other spice-related activities.
FDA has posted Questions & Answers on Improving the Safety of Spices. The Q&A addresses spice sampling conducted at the retail level, which is still being analyzed, in addition to sampling FDA has previously conducted at import. The Q&A also addresses how the FDA Food Safety Modernization Act rules are expected to improve spice safety and discusses other spice-related activities.

Center for Food Safety and Applied Nutrition
The Center for Food Safety and Applied Nutrition, known as CFSAN, carries out the mission of FDA. The Center provides services to consumers, domestic and foreign industry and other outside groups regarding field programs; agency administrative tasks; scientific analysis and support; and policy, planning and handling of critical issues related to food and cosmetics. More information
Food Facts for You
The Center for Food Safety and Applied Nutrition, known as CFSAN, issues food facts for consumers to keep you and your family safe. More information |
Hypothyroidism in Dogs—There’s now an FDA-Approved Treatment
Hypothyroidism occurs when the thyroid gland doesn’t produce and secrete enough thyroid hormones. The thyroid gland is located in the mid-neck region near the voice box (larynx). In dogs, the thyroid gland is made up of two separate lobes that lie on either side of the windpipe (trachea). Thyroid hormones play a big role in metabolism and affect the function of many parts of the body. There is no cure for hypothyroidism. Dogs must be treated for life with thyroid hormone replacement therapy. Only one drug,THYRO-TABS CANINE (levothyroxine sodium tablets, NADA2 141-448), is FDA-approved for replacement therapy for diminished thyroid function in dogs. Approved by FDA in January 2016 and manufactured by Lloyd, Inc., THYRO-TABS CANINE is a prescription drug containing levothyroxine sodium as the active ingredient. The tablets are available in nine strengths. Your veterinarian will carefully calculate the dose depending on your dog’s weight. The drug is given by mouth every 12 or 24 hours, as prescribed by your veterinarian. More information
Hypothyroidism occurs when the thyroid gland doesn’t produce and secrete enough thyroid hormones. The thyroid gland is located in the mid-neck region near the voice box (larynx). In dogs, the thyroid gland is made up of two separate lobes that lie on either side of the windpipe (trachea). Thyroid hormones play a big role in metabolism and affect the function of many parts of the body. There is no cure for hypothyroidism. Dogs must be treated for life with thyroid hormone replacement therapy. Only one drug,THYRO-TABS CANINE (levothyroxine sodium tablets, NADA2 141-448), is FDA-approved for replacement therapy for diminished thyroid function in dogs. Approved by FDA in January 2016 and manufactured by Lloyd, Inc., THYRO-TABS CANINE is a prescription drug containing levothyroxine sodium as the active ingredient. The tablets are available in nine strengths. Your veterinarian will carefully calculate the dose depending on your dog’s weight. The drug is given by mouth every 12 or 24 hours, as prescribed by your veterinarian. More information

Animal Health Literacy
Animal Health Literacy means timely information for the benefit of all animals and their humans. With continuous communication and outreach, the Center for Veterinary Medicine (CVM) strives to enhance the public trust, promote safe and effective use of the animal health products we regulate, and share our scientific endeavors. CVM provides reliable, science-based information to promote animal and human health. More information/Publicaciones en Español del
Animal and Veterinary Updates
Animal and veterinary updates provide information to keep your pets healthy and safe. More information |

How to Report a Pet Food Complaint
You can report complaints about a pet food product electronically through the Safety Reporting Portal or you can call your state’s FDA Consumer Complaint Coordinators. Please provide as much information as possible in your complaint, such as exact name of product, type of container, lot number, UPC codes, how the food was stored, and purchase date and exact location where purchased. If possible, please save the original packaging until the pet food has been consumed. The packaging contains IMPORTANT information often needed to identify the variety of pet food, the manufacturing plant, and the production date.More information |

Public Health Education
Tobacco products are harmful, yet widely used, consumer products that are responsible for severe health problems in both users and non-users. These health problems include cancer, lung disease, and heart disease, which often lead to death.
|
Public Education CampaignsWe are investing in a number of public education campaigns, such as Fresh Empire and The Real Cost, to help educate the public – especially youth – about the dangers of regulated tobacco products. Rooted in science, these efforts are directly linked to our authority to regulate the marketing and sales of tobacco products. More information
Youth and TobaccoWe are working to protect the health of America’s children and ultimately reduce the burden of illness and death caused by tobacco use. More information

Information about Expanded Access
Expanded access, sometimes called "compassionate use," is the use outside of a clinical trial of an investigational medical product (i.e., one that has not been approved by FDA). FDA is committed to increasing awareness of and knowledge about its expanded access programs and the procedures for obtaining access to human investigational drugs (including biologics) and medical devices. More information |
Learn about what your physician should do before submitting a request for individual patient expanded access use of an investigational medical product, who may be eligible for expanded access, associated costs, FDA contacts and more. Information for Patients
|
Learn about your responsibilities under the expanded access pathway, how to submit a request for expanded access for an individual patient (including for emergency use), which forms to use, FDA contacts and more. Information for Physicians
|

Patient Network Webinars
Through our webinars and presentations, the Office of Health and Constituent Affairs brings information to you on many topics related to patient engagement, medical product (Drugs, Biologics, Devices) approval and medical product safety updates. More information
FDA Basics
Each month, different centers and offices at FDA will host an online session where the public can ask questions to senior FDA officials about a specific topic or just listen in to learn more about FDA. More information
Educational Videos
|

healthfinder.gov
Welcome to healthfinder.gov, a government Web site where you will find information and tools to help you and those you care about stay healthy.More information /más información
FDA E-list
Sign up for one of the FDA disease specific e-mail list that delivers updates, including product approvals, safety warnings, notices of upcoming meetings, and notices on proposed regulatory guidances.
You may wish to sign up for other email updates from the FDA - see other email updates.
|
Patient Network - Bring Your Voice to FDA
An interactive tool for educating patients, patient advocates, and consumers on how their medications - both prescription and over-the-counter - and medical devices move from the realm of idea to the realm of the marketplace. More information |





















.png)












No hay comentarios:
Publicar un comentario