

Dexcom Inc. Recalls G4 Platinum and G5 Mobile Continuous Glucose Monitoring System Receivers Due to Audible Alarm FailureThe Dexcom Continuous Glucose Monitoring Systems are used to monitor the blood sugar (glucose) level of adult and pediatric patients with type 1 or type 2 diabetes. These glucose monitoring systems include a sensor that is placed under the skin to measure blood glucose readings that are sent to a hand-held receiver. They are used in combination with standard home glucose monitoring devices in the management of diabetes. More information.
|

Boston Scientific (NYSE: BSX) has initiated a global, voluntary recall of all models of its Fetch™ 2 Aspiration Catheter.
A thrombectomy catheter is used during procedures to remove small blood clots from coronary arteries. The Fetch 2 catheters were recalled on March 22, 2016, due to complaints of shaft breakage. The U.S. Food and Drug Administration (FDA) classified the action as a Class 1 recall. This recall designation means that the use of the device exposes the patient to a reasonable chance of a serious adverse health consequence or death. There have been no reports of patient injury or death, and there is no risk to patients who previously underwent a thrombectomy procedure with the Fetch 2 catheter. More information |

Interference between CT and Electronic Medical Devices
This website provides information about a rare and preventable type of interference between Computed Tomography (CT) and electronic medical devices. This information updates and replaces our 2008 preliminary public health notification. CT is a valuable type of diagnostic imaging. Most patients undergo CT scans without any adverse consequences. However, the FDA has received a small number of reports of adverse events that we believe to be associated with CT imaging of some implantable and wearable electronic devices (eg. insulin pumps, cardiac implantable electronic devices and neurostimulators). More information |

Urogynecologic Surgical Mesh Implants
The FDA is aware of allegations that Boston Scientific's urogynecologic surgical mesh may contain counterfeit raw material. We are examining these allegations to determine any necessary and appropriate next steps. We are currently not aware that the alleged counterfeit raw material contributes to adverse events associated with these products. It is not uncommon for a firm, based on its own appropriate evaluation of potential suppliers and raw material, to change the source of a raw material after the device has been cleared by the FDA, and such a change often does not require FDA premarket review. However, in light of the allegations, Boston Scientific will conduct additional testing relevant to the safety and effectiveness of the finished product. More information |

Diabetes Medications Containing Saxagliptin and Alogliptin: Drug Safety Communication - Risk of Heart Failure
An FDA safety review has found that type 2 diabetes medicines containing saxagliptin and alogliptin may increase the risk of heart failure, particularly in patients who already have heart or kidney disease. As a result, FDA is adding new warnings to the drug labels about this safety issue. Saxagliptin and alogliptin are part of the class of dipeptidyl peptidase-4 (DPP-4) inhibitor drugs, which are used with diet and exercise to lower blood sugar in adults with type 2 diabetes. More information |

FDA Drug Safety Communication: FDA revises warnings regarding use of the diabetes medicine metformin in certain patients with reduced kidney function
FDA is requiring labeling changes regarding the recommendations for metformin-containing medicines for diabetes to expand metformin’s use in certain patients with reduced kidney function. The current labeling strongly recommends against use of metformin in some patients whose kidneys do not work normally. Health care professionals should follow the latest recommendations when prescribing metformin-containing medicines to patients with impaired kidney function. Patients should talk to their health care professionals if they have any questions or concerns. More information |
Comunicaciones de la FDA sobre la seguridad de los medicamentos en español
Descargo de responsabilidad: La FDA reconoce la necesidad de proporcionar información importante sobre seguridad de los medicamentos en idiomas distintos al inglés. Hacemos lo mejor posible para proporcionar versiones en español precisas y oportunas de nuestras Comunicaciones de Seguridad de Medicamentos. Sin embargo, en caso que existiera discrepancias entre las versiones en inglés y la de español, la información contenida en la versión en inglés es la que se considera como versión oficial. Si tiene alguna pregunta, por favor contáctese con Division of Drug Information endruginfo@fda.hhs.gov. Comunicaciones de la FDA |


FDA recognizes the significant public health consequences that can result from drug shortages and takes tremendous efforts within its legal authority to address and prevent drug shortages. These shortages occur for many reasons, including manufacturing and quality problems, delays, and discontinuations. When issues are discovered by the company or the public and reported to FDA or are found by FDA upon inspection, FDA works closely with the firm to address risks involved to prevent harm to patients. FDA also considers the impact a shortage would have on patient care and access and works with the firm to restore supplies while also ensuring safety for patients. More information
|
Drug Shortages Voluntarily Reported by Manufacturers During the Past 2 Weeks:
Drug Shortages Reported to be Resolved by Manufacturers During the Past 2 Weeks:
La FDA reconoce las consecuencias significativas para la salud pública que pueden resultar de la escasez de medicamentos y hace un gran esfuerzo dentro de sus facultades legales para abordar yprevenir la escasez de medicamentos. La escasez se produce por muchas razones, incluyendoproblemas de fabricación y calidad, retrasos y discontinuación del producto. Cuando los problemas son descubiertos por la empresa o el público y reportados a la FDA o se descubren por inspecciones de la FDA, la FDA trabaja en estrecha colaboración con la empresa para hacer frente a los riesgosinvolucrados y evitar daños a los pacientes. La FDA también considera el impacto que una escaseztendría en la atención médica del paciente y al acceso del producto y trabaja con la empresa pararestablecer el suministro al tiempo que garantiza la seguridad de los pacientes. Más información
|

FDA approves first leadless pacemaker to treat heart rhythm disorders FDA approved the first pacemaker that does not require the use of wired leads to provide an electrical connection between the pulse-generating device and the heart. While the Micra Transcatheter Pacing System works like other pacemakers to regulate heart rate, the self-contained, inch-long device is implanted directly in the right ventricle chamber of the heart.
Pacemakers are surgically implanted medical devices that generate electrical impulses to treat irregular or stalled heart beats, and nearly 1 million people worldwide are implanted with pacemakers each year.More information
|
FDA approves Inflectra, a biosimilar to RemicadeFDA approved Inflectra (infliximab-dyyb) for multiple indications. Inflectra is administered by intravenous infusion.
Inflectra is biosimilar to Janssen Biotech, Inc.’s Remicade (infliximab), which was originally licensed in 1998. Inflectra is approved and can be prescribed by a health care professional. More informartion
|

The FDA approves Venetoclax, for patients with Chronic Lymphocitic LeukemiaFDA approved venetoclax (VENCLEXTA tablets, marketed by AbbVie, Inc. and Genentech USA, Inc.) for the treatment of patients with chronic lymphocytic leukemia (CLL) with 17p deletion, as detected by an FDA-approved test, who have received at least one prior therapy. More information
|

FDA allows marketing of first-of-kind tissue containment system for use with certain laparoscopic power morcellators in select patients
FDA permitted the marketing of PneumoLiner, the first tissue containment system for use with certain laparoscopic power morcellators to isolate uterine tissue that is not suspected to contain cancer. Although the device is an effective tissue containment system, the FDA is requiring the manufacturer to warn patients and health care providers that PneumoLiner has not been proven to reduce the risk of spreading cancer during these procedures. More information |
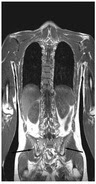
FDA approves first treatment for rare disease in patients who receive stem cell transplant from blood or bone marrow
FDA approved Defitelio (defibrotide sodium) to treat adults and children who develop hepatic veno-occlusive disease (VOD) with additional kidney or lung abnormalities after they receive a stem cell transplant from blood or bone marrow called hematopoietic stem cell transplantation (HSCT). This is the first FDA-approved therapy for treatment of severe hepatic VOD, a rare and life-threatening liver condition. More information |
For information on drug approvals or to view prescribing information and patient information, please visit Drugs@FDA or DailyMed.

View FDA's Comments on Current Draft Guidance page, for a list of current draft guidances and other topics of interest for patients and caregivers.
|
2015: An Important Year for Advancing Generic Drugs at FDA
The Office of Generic Drugs (OGD) in the Center for Drug Evaluation and Research, 2015 was an important year. It was our first full year of operation after vastly expanding our office’s scope and structure. This change allowed for the office to have greater prominence and allowed for additional staff to handle a growing workload and enhance our ability to advance the safety and availability of generic drugs in the U.S.Consider this: In 2014, generics saved the U.S. health system an estimated $254 billion – and FDA continues to work hard to advance the use of generic drugs to help improve public health. More information |  |

FDA Patient Representatives NeededThe FDA Patient Representative Program is managed by theOffice of Health and Constituent Affairs within the Office of the Commissioner. The Office of Health and Constituent Affairs-Patient Liaison Team coordinates the recruitment, training, and retention for over 200 FDA Patient Representatives, who are patients or primary caregivers to patients. These FDA Patient Representatives are knowledgeable and experienced in over 300 diseases and conditions and participate on 47 FDA Advisory Committees and panels, and in review division meetings. These Patient Representatives provide direct input to inform the Agency’s decision-making associated with medical products for drugs, biologics, and medical devices. For more information about the FDA Patient Representative Program and to see what qualifications are needed to become an FDA Patient Representative.
|
Minority Women’s Health Twitter ChatOn April 19, Join FDA’s Office of Women’s Health (@FDAWomen), FDA’s Office of Minority Health (@FDAOMH) and FDA en Español (@FDAenEspanol) for a #FDAHealthChat on Twitter on April 19 from 1-2 p.m. EDT.This bilingual chat will focus on tips minority women can use to live a healthier life. It will also highlight how to empower diverse women to take control of their health.
|
|

FDA allows use of investigational test to screen blood donations for Zika virus
FDA announced the availability of an investigational test to screen blood donations for Zika virus. The screening test may be used under an investigational new drug application (IND) for screening donated blood in areas with active mosquito-borne transmission of Zika virus. “The availability of an investigational test to screen donated blood for Zika virus is an important step forward in maintaining the safety of the nation’s blood supply, especially for those U.S. territories already experiencing active transmission,” said Peter Marks, M.D., Ph.D., director of the FDA’s Center for Biologics Evaluation and Research. “In the future, should Zika virus transmission occur in other areas, blood collection establishments will be able to continue to collect blood and use the investigational screening test, minimizing disruption to the blood supply.” More information |

Developing a Consensus Voice: The Combination Products Policy Council
FDA announced the launch of lean process mapping to build a better system for combination products review – one that is more cohesive, more collaborative, more systematic, and more predictable. We look forward to providing an update on this effort soon.In the meantime, we’re delighted to announce the creation of FDA’s first Combination Products Policy Council. Building on successful cross-cutting efforts such as the Biosimilars Implementation Committee and the Medical Policy Counsel in the Center for Drug Evaluation and Research (CDER), the Council will be a senior-level, agency-wide forum for discussing, resolving, and implementing product and policy issues. More information |
National Evaluating SystemThe FDA is building the foundations of a national evaluation system to generate better evidence more efficiently for medical device evaluation and regulatory decision-making. A national evaluation system would generate evidence across the total product lifecycle of medical devices by strategically and systematically leveraging real-world evidence, and applying advanced analytics to data tailored to the unique data needs and innovation cycles of medical devices. The collaborative national evaluation system will link and synthesize data from different sources across the medical device landscape, including clinical registries, electronic health records and medical billing claims. A national evaluation system will help improve the quality of real-world evidence that health care providers and patients can use to make better informed treatment decisions and strike the right balance between assuring safety and fostering device innovation and patient access. More information
FDA advisory committee meetings are free and open to the public. No prior registration is required to attend. Interested persons may present data, information, or views, orally at the meeting, or in writing, on issues pending before the committee.
Other types of meetings listed may require prior registration and fees.
View FDA's Calendar of Public Meetings page for a complete list of meetings and workshops.
- Calendar of FDA Sponsored Public Meetings - April 2016
- Calendar of FDA Sponsored Public Meetings - May 2016
- Calendar of FDA Sponsored Public Meetings - June 2016

Public Workshop: Developing an Expanded Access Navigator for Individual Patient Access to Investigational Drugs
Date: May 16, 2016 Time: 8:30 am to 11:45 am Agenda: A public workshop, co-hosted by the Reagan-Udall Foundation for the FDA and the Food and Drug Administration, to solicit feedback on a proposed Expanded Access Navigator. The objective of the meeting is to hold an open discussion with interested stakeholders on the concept of an Expanded Access Navigator. The intent of the EA Navigator is to provide a coordinated resource for healthcare providers and patients seeking information on the single-patient IND (expanded access/compassionate use) request process. The workshop presents an opportunity for the public to provide input on the design, necessity, and refinement of the Expanded Access Navigator proposal. Please register for this public workshop, to attend either in person or via web cast, before May 9, 2016. There is no fee to attend this workshop, but attendees should register in advance. Space is limited and will be on a first-come, first-served basis. Additional information about the meeting, including meeting materials, can be found at: http://bit.ly/1ULcAkO |

Public Workshop: Lactation
Date: April 27 and 28, 2016 Agenda: The purpose of this 2-day workshop is to provide a forum to discuss the current state and future directions of the collection of data on the potential risks to breastfed infants with maternal use of medications during lactation. More information |

The FDA Offices of Hematology and Oncology Products, and Health and Constituent Affairs invite you to participate in a FDA Outreach to the Pediatric Cancer Advocacy Community.
Date: Friday April 22, 2016 Time: 9:00 a.m. to 12:00 p.m. Topics for Discussion: Update of cancer drugs approved for pediatric use, BPCA/WR study results which have informed product labeling, PREA and iPSPs for oncology drugs- impact on issuance of WRs, Expanding patient-focused drug development to children with cancer and Pediatric PROs, Expanded Access to investigational drugs, Expanding Eligibility Criteria for clinical trials to accommodate early evaluation of certain products in children, and promising new Vaccine and Engineered Cell Products for cancer. |
Please visit FDA’s Advisory Committee page to obtain advisory committee meeting agendas, briefing materials, and meeting rosters prior to the meetings. You may also visit this page after meetings to obtain transcripts, presentations, and voting results. For additional information on other agency meetings please visit Meetings, Conferences, & Workshops.
|

Arsenic in RiceRice is not the only food or beverage that contains arsenic. It’s also found in vegetables, fruits, and many other foods. The FDA has been monitoring the presence of arsenic in food as part of its ongoing oversight of the safety of the food supply. And now we’ve looked at arsenic in infant rice cereal.
Arsenic is an element in the Earth’s crust and is present in very small amounts in water, soil and air. Crops absorb arsenic as they grow. That’s how it gets into foods and beverages — it’s not an additive or ingredient — and it cannot be completely eliminated. There are two forms of arsenic, organic and inorganic, with inorganic being the more toxic. The term “organic” in this case has nothing to do with types of farming. It’s about chemical elements. If arsenic atoms bond with carbon, the compound is organic. If there’s no carbon present, it’s inorganic. More information
|

Colorectal Cancer: What You Should KnowLast year in the United States, more than 136,000 people were diagnosed with—and more than 50,000 died from—colorectal cancer, according to the National Cancer Institute. It is the second leading cause of cancer-related deaths in the United States, striking some groups more often than others. The toll this disease takes on minorities is especially high, said Jonca Bull, M.D., director of FDA’s Office of Minority Health. Populations with limited access to screening and early treatment die much more often from the disease—African Americans, Hispanics, and American Indians and Alaska Natives. But there is a way of confronting this hazard, she added: “Early detection, referral, and treatment can significantly reduce disparities in deaths from colorectal cancer.” Colorectal cancer usually starts from polyps or other precancerous growths in the rectum or the colon (large intestine). People with precancerous growths or signs of colorectal cancer don’t always show symptoms. That’s why screening is important—doctors can see and remove growths or suspicious tissue before they become cancerous. More information
|

Healthy Breakfasts for Kids: It's All about BalanceA healthy breakfast is a must for kids. Skip it and your kids will be playing nutritional catch-up for the rest of the day, says Carole L. Adler, M.A., R.D., a dietitian at the Food and Drug Administration (FDA). When kids skip breakfast, they don't get what they need to be at their best, says Adler. “Growing bodies and developing brains need regular, healthy meals,” she says. According to the Academy of Nutrition and Dietetics, studies show that school children who eat breakfast perform better in the classroom. As with other meals, it’s a good idea for your kids (and you) to eat a healthy balance of fruits and vegetables, proteins, grains and dairy—not just for breakfast but throughout the day. Here are Adler’s seven quick and easy breakfast tips to ensure your children start their day off right. Anything goes, as long as you maintain a healthy balance. So if your kids want a change from cereal and eggs, think about serving left-overs from last night’s dinner. There’s nothing wrong with tuna fish with celery on a whole wheat English muffin or a turkey sandwich to start the day. More information
|
More Consumer Updates
For previously published Consumer Update articles that are timely and easy-to-read and cover all FDA activities and regulated products. More information
For previously published Consumer Update articles that are timely and easy-to-read and cover all FDA activities and regulated products. More information
En Español
La información en esta página es para el público en general, y para profesionales y educadores de salud. Esta información puede ser distribuida y publicada sin previa autorización. En Español
La información en esta página es para el público en general, y para profesionales y educadores de salud. Esta información puede ser distribuida y publicada sin previa autorización. En Español

Benefits Associated with Complete Adoption and Implementation of the FDA Food Code
A goal of the Food and Drug Administration’s (FDA) Retail Food Safety Initiative1 is to “Encourage widespread, uniform, and complete adoption of the FDA Food Code.” To address this goal, FDA emphasizes benefits that can be realized when State, territorial, local, and tribal governments adopt the Food Code in its entirety. Recognition of these benefits by the retail store, food service and vending industries should help to promote complete and widespread Food Code adoption as statutes, codes and ordinances pertaining to retail food safety are updated at all levels of government. More information |

WD Import and Export Inc. Issues an Alert on Dried Fish Due To Possible Health RiskWD Import and Export Inc. located at 4703 2nd Ave, Brooklyn, NY 11232 is recalling its bulk unlabeled cardboard boxes of Dried Yellow Fish because it has the potential to be contaminated with Clostridium botulinum, a bacterium which can cause life threatening illness or death. Consumers are warned not to use the product even if it does not look or smell spoiled. Botulism, a potential fatal form of food poisoning, can cause the following symptoms: general weakness, dizziness, blurred or double-vision and trouble with speaking or swallowing. Difficulty in breathing weakness of other muscles abdominal distention and constipation may also be common symptoms. People experiencing these problems should seek immediate medical attention. More information
|

FDA releases final rule to ensure food safety during transport
FDA finalized a new food safety rule under the landmark FDA Food Safety Modernization Act (FSMA) that will help to prevent food contamination during transportation. The rule will require those involved in transporting human and animal food by motor or rail vehicle to follow recognized best practices for sanitary transportation, such as properly refrigerating food, adequately cleaning vehicles between loads and properly protecting food during transportation. More information |

Methylsynephrine in Dietary Supplements
Methylsynephrine is a substance that does not meet the statutory definition of a dietary ingredient. The Federal Food, Drug, and Cosmetic Act defines a dietary ingredient as a vitamin; mineral; herb or other botanical; amino acid; dietary substance for use by man to supplement the diet by increasing the total dietary intake; or a concentrate, metabolite, constituent, extract, or combination of the preceding substances. Methylsynephrine does not fit under any of these categories, rendering misbranded any dietary supplement products that declare methylsynephrine as a dietary ingredient. |
 |
Center for Food Safety and Applied NutritionThe Center for Food Safety and Applied Nutrition, known as CFSAN, carries out the mission of FDA. The Center provides services to consumers, domestic and foreign industry and other outside groups regarding field programs; agency administrative tasks; scientific analysis and support; and policy, planning and handling of critical issues related to food and cosmetics. More information
Food Facts for YouThe Center for Food Safety and Applied Nutrition, known as CFSAN, issues food facts for consumers to keep you and your family safe. More information
|

FDA takes steps to withdraw approval of the swine drug carbadox due to safety concernsThe Food and Drug Administration’s Center for Veterinary Medicine (CVM) took the first step toward rescinding its approval of the use of carbadox to treat swine because the drug may leave trace amounts of a carcinogenic residue.CVM’s action comes after the center recently reexamined the safety profile of the drug and conducted a preliminary risk characterization that indicated there could be potential risk to human health from ingesting pork, especially pork liver, derived from carbadox-treated pigs. “The manufacturer of carbadox has failed to provide sufficient scientific data to demonstrate the safety of this drug given evidence that carbadox may result in carcinogenic residues,” said Michael R. Taylor, FDA deputy commissioner for foods and veterinary medicine. “As a result, FDA’s Center for Veterinary Medicine is taking legal action to remove this product from the marketplace.” The FDA is not recommending that people make changes in their food choices while the agency is working to remove carbadox from the market. More information
|

Animal Health Literacy
Animal Health Literacy means timely information for the benefit of all animals and their humans. With continuous communication and outreach, the Center for Veterinary Medicine (CVM) strives to enhance the public trust, promote safe and effective use of the animal health products we regulate, and share our scientific endeavors. CVM provides reliable, science-based information to promote animal and human health. More information and Publicaciones en Español del
Animal and Veterinary Updates
Animal and veterinary updates provide information to keep your pets healthy and safe. More information |

How to Report a Pet Food Complaint
You can report complaints about a pet food product electronically through the Safety Reporting Portal or you can call your state’s FDA Consumer Complaint Coordinators. Please provide as much information as possible in your complaint, such as exact name of product, type of container, lot number, UPC codes, how the food was stored, and purchase date and exact location where purchased. If possible, please save the original packaging until the pet food has been consumed. The packaging contains IMPORTANT information often needed to identify the variety of pet food, the manufacturing plant, and the production date. More information |

Public Health EducationTobacco products are harmful, yet widely used, consumer products that are responsible for severe health problems in both users and non-users. These health problems include cancer, lung disease, and heart disease, which often lead to death.
Access the latest Health Information available from our Federal partners. Learn about Tobacco-Related Health Fraud.
|
Public Education CampaignsWe are investing in a number of public education campaigns, such as Fresh Empire and The Real Cost, to help educate the public – especially youth – about the dangers of regulated tobacco products. Rooted in science, these efforts are directly linked to our authority to regulate the marketing and sales of tobacco products. More information
Youth and TobaccoWe are working to protect the health of America’s children and ultimately reduce the burden of illness and death caused by tobacco use. More information

Information about Expanded AccessExpanded access, sometimes called "compassionate use," is the use outside of a clinical trial of an investigational medical product (i.e., one that has not been approved by FDA). FDA is committed to increasing awareness of and knowledge about its expanded access programs and the procedures for obtaining access to human investigational drugs (including biologics) and medical devices. More information
|
Learn about what your physician should do before submitting a request for individual patient expanded access use of an investigational medical product, who may be eligible for expanded access, associated costs, FDA contacts and more.Information for Patients
|
Learn about your responsibilities under the expanded access pathway, how to submit a request for expanded access for an individual patient (including for emergency use), which forms to use, FDA contacts and more. Information for Physicians
|

FDA Acronyms and Abbreviations
The FDA Acronyms and Abbreviations database provides a quick reference to acronyms and abbreviations related to Food and Drug Administration (FDA) activities. The emphasis is on scientific, regulatory, government agency, and computer application terms. The database includes some FDA organizational and program acronyms. We have included some terms that are not strictly acronyms because of their relevance to FDA activities. Acronyms for journal titles do not appear in the database. More information |

Minority Health Month ResourcesLook to FDA for resources to support your outreach to women of all diverse cultural backgrounds.
Download women’s health materials in English, Spanish and 16 other languages.
Check out FDA’s Minority Health webpage. Join the Pink Ribbon campaign and educate your community about mammograms. Watch ¡Nunca Mas! novela series and teach women about safe medication use. Visit FDA en Español webpage. |

Patient Network WebinarsThrough our webinars and presentations, the Office of Health and Constituent Affairs brings information to you on many topics related to patient engagement, medical product (Drugs, Biologics, Devices) approval and medical product safety updates. More information
FDA BasicsEach month, different centers and offices at FDA will host an online session where the public can ask questions to senior FDA officials about a specific topic or just listen in to learn more about FDA. More information
Educational Videos
|

healthfinder.gov
Welcome to healthfinder.gov, a government Web site where you will find information and tools to help you and those you care about stay healthy.More information /más información
FDA E-list
Sign up for one of the FDA disease specific e-mail list that delivers updates, including product approvals, safety warnings, notices of upcoming meetings, and notices on proposed regulatory guidances.
You may wish to sign up for other email updates from the FDA - see other email updates.
|
Patient Network - Bring Your Voice to FDA
An interactive tool for educating patients, patient advocates, and consumers on how their medications - both prescription and over-the-counter - and medical devices move from the realm of idea to the realm of the marketplace. More information |




































No hay comentarios:
Publicar un comentario