

New Look
We have updated our look, but don't worry you will still find all of the important information you have come to rely on. The FDA Patient Network News, a bi-weekly newsletter provided by the FDA Office of Health and Constituent Affairs is intended to inform you of:
|

MedWatch Safety Alert: WEN by Chaz Dean Cleansing Conditioners: FDA Statement - Investigation of Adverse Event Reports
The FDA is investigating reports of hair loss, hair breakage, balding, itching, and rash associated with the use of WEN by Chaz Dean Cleansing Conditioner products.
While the FDA continues its investigation, consumers should be aware of reactions reported in association with the use of WEN by Chaz Dean Cleansing Conditioner products. Consumers who experience a reaction after using WEN by Chaz Dean Cleansing Conditioner products should stop using the product and consult with their dermatologist or other health care provider. The agency also urges consumers to report to FDA any reactions they may have experienced when using these products. More information
|

MedWatch Safety Alert: Oral Liquid Docusate Sodium by PharmaTech : Recall - Contaminated with B. Cepacia
The FDA is alerting health care professionals that PharmaTech LLC, Davie, Florida, is voluntarily recalling all non-expired lots of Diocto Liquid, a docusate sodium solution distributed by Rugby Laboratories, Livonia, Michigan. The agency confirmed the product has been contaminated with Burkholderia cepacia, a bacteria linked to an outbreak in five states.
In addition, FDA has received several adverse event reports of B. cepacia infections in patients. Some of these reports identify liquid docusate sodium products manufactured by companies other than PharmaTech. FDA and the Centers for Disease Control and Prevention continue to investigate the extent of this issue in order to identify other potentially contaminated liquid docusate sodium products. More information
|
Comunicaciones de la FDA sobre la seguridad de los medicamentos en español
Descargo de responsabilidad: La FDA reconoce la necesidad de proporcionar información importante sobre seguridad de los medicamentos en idiomas distintos al inglés. Hacemos lo mejor posible para proporcionar versiones en español precisas y oportunas de nuestras Comunicaciones de Seguridad de Medicamentos. Sin embargo, en caso que existiera discrepancias entre las versiones en inglés y la de español, la información contenida en la versión en inglés es la que se considera como versión oficial. Si tiene alguna pregunta, por favor contáctese con Division of Drug Information en druginfo@fda.hhs.gov.Comunicaciones de la FDA |


FDA knows the major public health consequences that can result from drug shortages. These shortages occur for many reasons including manufacturing and quality problems, delays and discontinuations.
When issues are discovered, FDA works closely with the company to address risks involved to prevent harm to patients. FDA also considers the impact a shortage would have on patient care and access and works with the company to restore supplies while also ensuring safety for patients. More information
|
Drug Shortages Voluntarily Reported by Manufacturers During the Past 2 Weeks:
Information about blood and biologic shortages, resolved shortages, and discontinuations
La FDA reconoce las consecuencias significativas para la salud pública que pueden resultar de la escasez de medicamentos y hace un gran esfuerzo dentro de sus facultades legales para abordar yprevenir la escasez de medicamentos. La escasez se produce por muchas razones, incluyendoproblemas de fabricación y calidad, retrasos y discontinuación del producto. Cuando los problemas son descubiertos por la empresa o el público y reportados a la FDA o se descubren por inspecciones de la FDA, la FDA trabaja en estrecha colaboración con la empresa para hacer frente a los riesgosinvolucrados y evitar daños a los pacientes. La FDA también considera el impacto que una escaseztendría en la atención médica del paciente y al acceso del producto y trabaja con la empresa pararestablecer el suministro al tiempo que garantiza la seguridad de los pacientes. Más información
|

First intraocular lens with extended range of vision approved for cataract patients
FDA approved the first intraocular lens (IOL) that provides cataract patients with an extended depth-of-focus, which helps improve their sharpness of vision (visual acuity) at near, intermediate and far distances.
Cataracts are a common eye condition where the natural lens becomes clouded, impairing a patient’s vision. According to the National Eye Institute, more than 20 percent of Americans will have cataracts by the age of 65, and the prevalence increases with age. In cataract surgery, the clouded natural lens is removed and replaced with an IOL. More information
|

New medication for dry eye disease approved
FDA approved Xiidra (lifitegrast ophthalmic solution) for the treatment of signs and symptoms of dry eye disease, on Monday, July 11, 2016. Xiidra is the first medication in a new class of drugs, called lymphocyte function-associated antigen 1 (LFA-1) antagonist, approved by the FDA for dry eye disease.
“Normal tear production is needed for clear vision and eye health,” said Edward Cox, M.D., director of the Office of Antimicrobial Products in the FDA’s Center for Drug Evaluation and Research. “This approval will provide a new treatment option for patients with dry eye disease.” More information
|

First MRI-guided focused ultrasound device approved to treat essential tremor
FDA approved the first focused ultrasound device to treat essential tremor in patients who have not responded to medication. ExAblate Neuro uses magnetic resonance (MR) images taken during the procedure to deliver focused ultrasound to destroy brain tissue in a tiny area thought to be responsible for causing tremors.
“Patients with essential tremor who have not seen improvement with medication now have a new treatment option that could help them to avoid more invasive surgical treatments,” said Carlos Peña, Ph.D., M.S., director of the division of neurological and physical medicine devices in the FDA’s Center for Devices and Radiological Health. “As with other treatments for essential tremor, this new device is not a cure but could help patients enjoy a better quality of life.” More information
|

Differin Gel 0.1% approved for over-the-counter use to treat acneFDA approved Differin Gel 0.1% (adapalene), a once-daily topical gel for the over-the-counter (OTC) treatment of acne. Differin Gel 0.1% is approved for use in people 12 years of age and older.
Differin Gel 0.1% is the first in a class of drugs known as retinoids to be made available OTC for the treatment of acne, and contains the first new active ingredient for acne treatment for OTC use since the 1980s. Differin Gel 0.1% was originally approved in 1996 as a prescription product for the treatment of acne vulgaris in patients 12 years of age and older. More information
|

First HPV test approved for use with SurePath Preservative FluidFDA approved the Roche cobas HPV Test as the first test for Human Papilloma Virus (HPV) that can be used with cervical cells obtained for a Pap test and collected in SurePath Preservative Fluid.
The FDA approves HPV tests to be used with specific collection fluid, which store and preserve cervical cell samples for testing in the laboratory. Until today, the FDA had not approved any HPV tests to be used with SurePath Preservative Fluid, one of two approved liquid collection fluids commonly used for Pap tests. More information
|
For information on drug approvals or to view prescribing information and patient information, please visit Drugs@FDA or DailyMed.

View FDA's Comments on Current Draft Guidance page, for a list of current draft guidances and other topics of interest for patients and caregivers.
|

Webinar: Next Generation Sequencing (NGS) Draft Guidances: Implications for Patients and Providers
Date: July 27, 2016 Time: 1:30 pm to 2:30 pm ET
Agenda: The FDA will host a webinar about draft guidances released July 6, which propose methods to streamline oversight of Next Generation Sequencing (NGS)-based tests. These two draft guidances are part of the FDA’s participation in President Obama’s Precision Medicine Initiative (PMI), which aims to take advantage of the progress made in genomic testing to accelerate the development of new treatments that take into account individual differences in people’s genes, environments, and lifestyles. More information
|

The Rise in Orphan Drug Designations: Meeting the Growing Demand, byGayatri Rao, M.D., J.D., is FDA’s Director for The Office of Orphan Products Development
Developing drugs for rare diseases, once considered a rare phenomenon itself, has fast become a mainstay for many companies’ drug development pipelines. This is exciting news for the 30 million Americans with rare diseases and their families.
Congress played no small role in making this a reality when it passed the Orphan Drug Act in 1983. One of the key features of this Act was the creation of the Orphan Drug Designation Program, which provides important financial incentives to encourage companies to develop drugs and biologics for rare diseases. This legislation includes major tax credits to defray the cost of conducting clinical trials, as well as eligibility for seven years of market exclusivity. As a result of later amendments to the Act, no user fee is required for orphan drug product submissions, except when an application includes an indication for a non-rare disease or condition.
To read the rest of this post, see FDA Voice on July 18, 2016
|

Learn About Patient Engagement at the FDA
The FDA has a difficult task when it comes to evaluating and approving new and innovative medical products.
Individual patients may experience the effects of diseases and therapies differently and each individual patient has a unique perspective about treatments or diagnostic procedures that differ from those perspectives of other patients or of their healthcare provider. The FDA has included the patient perspective in FDA Advisory Committee meetings since 1991. More informationabout the different opportunities that patient and caregivers can get involved in at the FDA.
|

CDER Director's Corner
Celebrating 30 years at FDA. Dr. Woodcock reflects on breakthroughs in drug research and regulation and makes some predictions for the future of drug products. Download Podcast orread transcript |

FDA advances Precision Medicine Initiative by issuing draft guidances on next generation sequencing-based tests
In support of the President’s Precision Medicine Initiative, FDA issued two draft guidances:
When finalized they will provide a flexible and streamlined approach to the oversight of tests that detect medically important differences in a person’s genomic makeup.
The powerful new technology, known as next generation sequencing (NGS), can scan a person’s DNA to detect genomic variations that may determine whether a person has or is at risk of disease or may help to inform treatment decisions. While current regulatory approaches are appropriate for conventional diagnostics that measure a limited number of substances associated with a disease or condition, such as blood glucose or cholesterol levels, the new sequencing technologies can examine millions of DNA variants at a time, and thus require a flexible approach to oversight that is adapted to the novel nature of these tests. More information
|

In this section you will find a comprehensive list of all the meetings that the FDA is involved with. The meetings may include advisory committee meetings, public workshops and public conferences that are seeking to hear from patients and caregivers.
Most FDA meetings are free to the public and do not require the public to register. Interested persons may present data, information, or views, orally at the meeting, or in writing, on issues pending before the committee. Other types of meetings listed may require prior registration and fees.
|
View FDA's Patient Network Calendar of Public Meetings page for a complete list of meetings and workshops.
For additional information on other agency meetings please visit Meetings, Conferences, & Workshops.
|

You May Be Surprised by How Much Salt You're Eating
Do you try to be careful about the amount of salt in your diet? Are you pretty sure you’re eating about the right amount of salt (also known as sodium chloride) every day, according to what most experts recommend?
You may be wrong about that.
Even if you throw your salt shaker away, you may still be taking in a lot of sodium — especially if you eat processed or prepared foods. More information
|
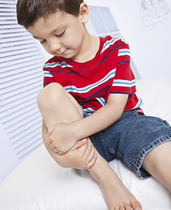
Juvenile Arthritis: Discoveries Lead to Newer Treatments
Arthritis is a disease that mostly affects older people, right? Not necessarily.
Juvenile arthritis is one of the most common chronic illnesses affecting children. In fact, nearly 300,000 youngsters nationwide have been diagnosed with the disease. The most common symptoms include joint pain, inflammation (swelling), tenderness and stiffness. One early sign may be limping in the morning.
Nikolay Nikolov, a rheumatologist and clinical team leader at the Food and Drug Administration (FDA), says that children with juvenile arthritis and their parents have reason to be optimistic. In the last several years, new therapies have been developed by drug companies and approved by the FDA that moderate the effects and control the disease, likely preventing significant disability in later years. More information
|
More Consumer Updates
For previously published Consumer Update articles that are timely and easy-to-read and cover all FDA activities and regulated products. More information
For previously published Consumer Update articles that are timely and easy-to-read and cover all FDA activities and regulated products. More information
En Español
La información en esta página es para el público en general, y para profesionales y educadores de salud. Esta información puede ser distribuida y publicada sin previa autorización. En Español
La información en esta página es para el público en general, y para profesionales y educadores de salud. Esta información puede ser distribuida y publicada sin previa autorización. En Español

Federal court orders Alabama seafood company to cease production due to food safety violations
The U.S. District Court for the Southern District of Alabama entered a consent decree of permanent injunction between the United States and BEK Catering LLC. The business, owned by Billy B. Stembridge, Jr. and Kyle D. Huxen, operates as Floppers Foods selling ready-to-eat seafood products and is based in Daphne, Alabama.
The consent decree was sought after the FDA documented serious violations of the seafood Hazard Analysis and Critical Control Point (HACCP) and of Current Good Manufacturing Practice regulations for foods. The consent decree prohibits BEK Catering, LLC, from receiving, processing, manufacturing, preparing, packing, holding or distributing food until it comes into compliance with FDA requirements.More information
|

Federal court orders Minnesota sprout and noodle company to cease operations due to unsanitary conditions
On July 15, 2016, the U.S. District Court for the District of Minnesota entered a consent decree of permanent injunction between the United States and Kwong Tung Foods, Inc., doing business as Canton Foods; its president and owner, Vieta “Victor” C. Wang; and its vice-president, Juney H. Wang, for significant and ongoing violations of the Federal Food, Drug, and Cosmetic Act (FD&C Act) and its implementing regulations. The business, located in Minneapolis, Minnesota, sells rice and wheat noodles, and mung bean and soy bean sprouts. More information |

Center for Food Safety and Applied Nutrition
The Center for Food Safety and Applied Nutrition, known as CFSAN, carries out the mission of FDA. The Center provides services to consumers, domestic and foreign industry and other outside groups regarding field programs; agency administrative tasks; scientific analysis and support; and policy, planning and handling of critical issues related to food and cosmetics. More information
Food Facts for You
The Center for Food Safety and Applied Nutrition, known as CFSAN, issues food facts for consumers to keep you and your family safe. More information |

Animal Health Literacy
Animal Health Literacy means timely information for the benefit of all animals and their humans. With continuous communication and outreach, the Center for Veterinary Medicine (CVM) strives to enhance the public trust, promote safe and effective use of the animal health products we regulate, and share our scientific endeavors. CVM provides reliable, science-based information to promote animal and human health. More information and Publicaciones en Español del
Animal and Veterinary Updates
Animal and veterinary updates provide information to keep your pets healthy and safe. More information |

How to Report a Pet Food Complaint
You can report complaints about a pet food product electronically through the Safety Reporting Portal or you can call your state’s FDA Consumer Complaint Coordinators. Please provide as much information as possible in your complaint, such as exact name of product, type of container, lot number, UPC codes, how the food was stored, and purchase date and exact location where purchased. If possible, please save the original packaging until the pet food has been consumed. The packaging contains IMPORTANT information often needed to identify the variety of pet food, the manufacturing plant, and the production date.More information |

Public Health Education
Tobacco products are harmful, yet widely used, consumer products that are responsible for severe health problems in both users and non-users. These health problems include cancer, lung disease, and heart disease, which often lead to death.
|
Public Education CampaignsWe are investing in a number of public education campaigns, such as Fresh Empire and The Real Cost, to help educate the public – especially youth – about the dangers of regulated tobacco products. Rooted in science, these efforts are directly linked to our authority to regulate the marketing and sales of tobacco products. More information
Youth and TobaccoWe are working to protect the health of America’s children and ultimately reduce the burden of illness and death caused by tobacco use. More information

What is a Cosmetic?
The Federal Food, Drug, and Cosmetic Act (FD&C Act) defines cosmetics by their intended use, as "articles intended to be rubbed, poured, sprinkled, or sprayed on, introduced into, or otherwise applied to the human body...for cleansing, beautifying, promoting attractiveness, or altering the appearance" [FD&C Act, sec. 201(i)]. Among the products included in this definition are skin moisturizers, perfumes, lipsticks, fingernail polishes, eye and facial makeup preparations, cleansing shampoos, permanent waves, hair colors, and deodorants, as well as any substance intended for use as a component of a cosmetic product.More information |
How to Report a Cosmetic Related Problem
You can report a problem you have experienced with a cosmetic to FDA's MedWatch online or by call 1-800-FDA-1088.
You can also contact the FDA district office consumer complaint coordinator for your geographic area.
You can report a problem you have experienced with a cosmetic to FDA's MedWatch online or by call 1-800-FDA-1088.
You can also contact the FDA district office consumer complaint coordinator for your geographic area.
Recalls and Alerts
To see safety alerts and recent recalls related to cosmetics and other products regulated by FDA. More information
To see safety alerts and recent recalls related to cosmetics and other products regulated by FDA. More information

Information about Expanded Access
Expanded access, sometimes called "compassionate use," is the use outside of a clinical trial of an investigational medical product (i.e., one that has not been approved by FDA). FDA is committed to increasing awareness of and knowledge about its expanded access programs and the procedures for obtaining access to human investigational drugs (including biologics) and medical devices. More information |
Learn about what your physician should do before submitting a request for individual patient expanded access use of an investigational medical product, who may be eligible for expanded access, associated costs, FDA contacts and more. Information for Patients
|
Learn about your responsibilities under the expanded access pathway, how to submit a request for expanded access for an individual patient (including for emergency use), which forms to use, FDA contacts and more. Information for Physicians
Download FDA Form 3926 - Microsoft Explorer is the recommended browser to open this form.
|

Patient Network Webinars
Through our webinars and presentations, the Office of Health and Constituent Affairs brings information to you on many topics related to patient engagement, medical product (Drugs, Biologics, Devices) approval and medical product safety updates. More information
FDA Basics
Each month, different centers and offices at FDA will host an online session where the public can ask questions to senior FDA officials about a specific topic or just listen in to learn more about FDA. More information
Educational Videos
|

healthfinder.gov
Welcome to healthfinder.gov, a government Web site where you will find information and tools to help you and those you care about stay healthy.More information /más información
FDA E-list
Sign up for one of the FDA disease specific e-mail list that delivers updates, including product approvals, safety warnings, notices of upcoming meetings, and notices on proposed regulatory guidances.
You may wish to sign up for other email updates from the FDA - see other email updates.
|






















.png)











No hay comentarios:
Publicar un comentario