

Bioengineers grow living bone for facial reconstructionAugust 4, 2016 - Columbia University
Bioengineers grow living bone for facial reconstruction
Integration of engineered cells with natural bone matrix showed critical advances in a pig model study
Researchers have engineered living bone tissue to repair bone loss in the jaw, a structure that is typically difficult to restore. The team led by researchers from Columbia University, New York, grafted customized implants into pig jaws that resulted in integration and function of the engineered graft into the recipient’s own tissue.
The work, supported in part by the National Institute of Biomedical Imaging and Bioengineering (NIBIB), and reported in the June 15, 2016 issue of Science Translational Medicine, suggests that personalized bone grafts for facial reconstruction may be possible in the future.

Engineered jaw bone is transplanted to pigs. The engineered bone is cultivated from the pig's own stem cells seeded on ascaffold of cow-bone matrix.
Some current treatments replace missing bone with metal, bone putty derived from deceased donors, or grafted bone from elsewhere within a person’s own body. While using the patient’s own tissue is the safest strategy, sacrificing bone from elsewhere causes a secondary injury. It also can be difficult to obtain a piece large enough to carve into an exact match. Bone putty can be very useful to fill a gap, but is not structurally stable without reinforcements that doctors must remove in subsequent surgeries. Researchers have also experimented with implanting synthetic scaffolds that can enhance new bone growth, but these have not always been successful. To date, none of these options is as useful as doctors would like.
Researchers chose pigs to test the new bone reconstruction technique because their jaw anatomy and type of force and movement are similar to that of people—the ultimate beneficiaries of the potential treatment. They replaced a piece of the pigs’ jawbone with cow bone matrix—the material left after removing its resident cells. This matrix served as a scaffold for the graft. Guided by precise imaging technology, the researchers individually shaped each cow bone scaffold to fit perfectly into the missing jaw-bone region.
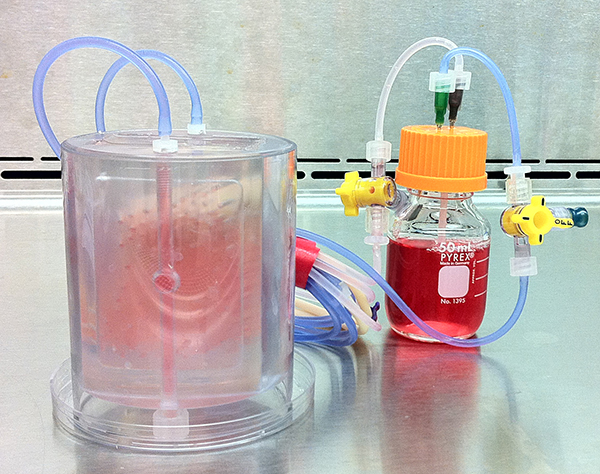
Researchers seeded a scaffold of cow-bone matrix with pig stem cells. They cultivated the engineered tissue in abioreactor device, pictured, transplanting the new bone after three weeks.
The researchers found that the engineered implants remodeled like natural bone. The team found that the pig’s body reacted to the implant just like its own bone, breaking it down and rebuilding it as needed.
“This was an unexpected and also very exciting finding because it tells us that these bones will really become an integral part of the body and continue to change in the body as we change,” said Gordana Vunjak-Novakovic, Ph.D., a professor of biomedical engineering and medical sciences at Columbia University and senior author of the paper. “It’s important that the implant is responding to these changes, and acting as an integral part of the native bone of the recipient.”
Six pigs received engineered implants, while six others received the cow bone without any stem cells. Two jaw-bone deficient pigs received no implant at all. At the six-month point, all the animals had some regrowth of the missing jaw. The researchers used quantitative image analysis to determine that those pigs with the engineered implant grew the most bone. In addition, their jaws could withstand the physical forces sustained during the animals’ activities.
Although the study was in pigs, the team performed procedures just as they would be done in humans, and are hopeful that the technique will translate to the clinic. “It’s never possible to say exactly, but we are aiming to get into clinical trials within the next few years,” said Vunjak-Novakovic. “Hopefully things continue to go well.”
“This is a promising step towards creating better implants for humans,” said Rosemarie Hunziker, Ph.D., director of the NIBIB program in Tissue Engineering. “The general size and mechanics of a pig’s jaw is a reasonable test of whether such a bone graft would integrate and do the work that would be required of a jaw bone graft in our human craniofacial system.”
Several key features enhanced the clinical prospects of this research, according to Hunziker. First, the team isolated the stem cells from fat, which can be harvested with less discomfort than from bone marrow, and yields ample cells due to abundant fat supply. They also avoided using growth factors—proteins that may improve bone quality but sometimes lead to excess bone growth, and would greatly increase the cost of the procedure. She attributed the researchers’ success to their understanding and integration of key factors: a well-prepared scaffold, the right cells, optimal growth conditions, and exact timing.
The work was a collaboration between researchers from the department of biomedical engineering and the division of oral and maxillofacial surgery at Columbia University in New York, New York; the School of Veterinary Medicine at Louisiana State University in Baton Rouge; LaCell LLC in New Orleans, Louisiana; and the Center for Stem Cell Research and Regenerative Medicine at Tulane University in New Orleans.
The research was funded in part by NIBIB (EB 002520) and the National Institute of Dental and Craniofacial Research (DE 016525), both parts of the National Institutes of Health.
Tissue-engineered autologous grafts for facial bone reconstruction. Bhumiratana S, Bernhard JC, Alfi DM, Yeager K, Eton RE, Bova J, Shah F, Gimble JM, Lopez MJ, Eisig SB, Vunjak-Novakovic G. Sci Transl Med. 2016 Jun 15.
—Teal Burrell, special to NIBIB
Program Area:
Health Terms:






















.png)











No hay comentarios:
Publicar un comentario