

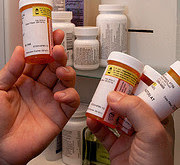
FDA requires strong warnings for opioid analgesics, prescription opioid cough products, and benzodiazepine labeling related to serious risks and death from combined use
After an extensive review of the latest scientific evidence, the FDA announced that it is requiring class-wide changes to drug labeling, including patient information, to help inform health care providers and patients of the serious risks associated with the combined use of certain opioid medications and a class of central nervous system (CNS) depressant drugs called benzodiazepines.
Among the changes, the FDA is requiring boxed warnings – the FDA’s strongest warning – and patient-focused Medication Guides for prescription opioid analgesics, opioid-containing cough products, and benzodiazepines – nearly 400 products in total – with information about the serious risks associated with using these medications at the same time. Risks include extreme sleepiness, respiratory depression, coma and death. Today’s actions are one of a number of steps the FDA is taking as part of the agency’s Opioids Action Plan, which focuses on policies aimed at reversing the prescription opioid abuse epidemic, while still providing patients in pain access to effective and appropriate pain management.More information
|

FDA issues final rule on safety and effectiveness of antibacterial soaps
The FDA issued a final rule establishing that over-the-counter (OTC) consumer antiseptic wash products containing certain active ingredients can no longer be marketed. Companies will no longer be able to market antibacterial washes with these ingredients because manufacturers did not demonstrate that the ingredients are both safe for long-term daily use and more effective than plain soap and water in preventing illness and the spread of certain infections. Some manufacturers have already started removing these ingredients from their products. This final rule applies to consumer antiseptic wash products containing one or more of 19 specific active ingredients, including the most commonly used ingredients – triclosan and triclocarban. These products are intended for use with water, and are rinsed off after use. This rule does not affect consumer hand “sanitizers” or wipes, or antibacterial products used in health care settings. More information |

United Exchange Corp Issues Voluntary Nationwide Recall of Family Care Brand Eye Wash Due to Microbial Contamination
Livonia, MI - United Exchange Corp. of Cerritos, CA, a primary source vendor of the Rugby®-branded Eye Irrigating Solution and Major®-branded Eye Wash, is voluntarily recalling those lots and expiration dates due to microbial contamination. These products consist of a purified water solution. Use of a contaminated product could be calamitous for any population since there is a reasonable probability of a potentially sight-threatening eye infection. Eye Wash/Eye Irrigating Solution is used to flush the eye to relieve irritation, stinging, or itching by removing foreign material such as air pollutants or chlorinated water. More information |

Novo Nordisk Inc. issues voluntary nationwide recall of six batches of GlucaGen® HypoKit® (glucagon [rDNA origin] for injection) due to detached needles on the syringe in the kit
Plainsboro, NJ - Novo Nordisk Inc. is recalling six batches of the GlucaGen® HypoKit® in the U.S. due to two customer complaints from the UK and Portugal involving detached needles on the syringe with Sterile Water for Injection (SWFI). GlucaGen® HypoKit® is indicated for the treatment of severe hypoglycemia (low blood sugar) in patients with diabetes who are treated with insulin. A syringe with a detached needle cannot be used as prescribed.
Untreated hypoglycemia can eventually lead to unconsciousness and seizures, which can prove fatal. If the blood glucose levels are not quickly restored, continuing hypoglycemia can lead to a decline in brain glucose levels which manifests through a variety of symptoms including cognitive dysfunction, sweating, tremors, convulsion and eventually coma or death. More information
|
Comunicaciones de la FDA sobre la seguridad de los medicamentos en español
Descargo de responsabilidad: La FDA reconoce la necesidad de proporcionar información importante sobre seguridad de los medicamentos en idiomas distintos al inglés. Hacemos lo mejor posible para proporcionar versiones en español precisas y oportunas de nuestras Comunicaciones de Seguridad de Medicamentos. Sin embargo, en caso que existiera discrepancias entre las versiones en inglés y la de español, la información contenida en la versión en inglés es la que se considera como versión oficial. Si tiene alguna pregunta, por favor contáctese con Division of Drug Information endruginfo@fda.hhs.gov. Comunicaciones de la FDA |


FDA knows the major public health consequences that can result from drug shortages. These shortages occur for many reasons including manufacturing and quality problems, delays and discontinuations. When issues are discovered, FDA works closely with the company to address risks involved to prevent harm to patients. FDA also considers the impact a shortage would have on patient care and access and works with the company to restore supplies while also ensuring safety for patients. More information
|
Drug Shortages Voluntarily Reported by Manufacturers During the Past 2 Weeks:
Drug Discontinuation Voluntarily Reported by Manufacturers During the Past 2 Weeks:
Information about blood and biologic shortages, resolved shortages, and discontinuations
La FDA reconoce las consecuencias significativas para la salud pública que pueden resultar de la escasez de medicamentos y hace un gran esfuerzo dentro de sus facultades legales para abordar yprevenir la escasez de medicamentos. La escasez se produce por muchas razones, incluyendoproblemas de fabricación y calidad, retrasos y discontinuación del producto. Cuando los problemas son descubiertos por la empresa o el público y reportados a la FDA o se descubren por inspecciones de la FDA, la FDA trabaja en estrecha colaboración con la empresa para hacer frente a los riesgosinvolucrados y evitar daños a los pacientes. La FDA también considera el impacto que una escaseztendría en la atención médica del paciente y al acceso del producto y trabaja con la empresa pararestablecer el suministro al tiempo que garantiza la seguridad de los pacientes. Más información
|

FDA approves VisuMax Femtosecond Laser to surgically treat nearsightednessThe FDA approved the VisuMax Femtosecond Laser for the small incision lenticule extraction (SMILE) procedure to reduce or eliminate nearsightedness in certain patients 22 years of age or older.
Not all patients are candidates for SMILE, and individuals should carefully review the patient labeling and discuss their expectations with their eye care professional. “This approval expands the surgical treatment options available to patients for correcting nearsightedness,” said Malvina Eydelman, M.D., director of Ophthalmic and Ear, Nose and Throat Devices, in FDA’s Center for Devices and Radiological Health. More information |
FDA approves Erelzi, a biosimilar to Enbrel
The FDA approved Erelzi, (etanercept-szzs) for multiple inflammatory diseases. Erelzi is a biosimilar to Enbrel (etanercept), which was originally licensed in 1998.
The FDA approved Erelzi, (etanercept-szzs) for multiple inflammatory diseases. Erelzi is a biosimilar to Enbrel (etanercept), which was originally licensed in 1998.
Erelzi is administered by injection for the treatment of:
- moderate to severe rheumatoid arthritis, either as a standalone therapy or in combination with methotrexate (MTX);
- moderate to severe polyarticular juvenile idiopathic arthritis in patients ages two and older;
- active psoriatic arthritis, including use in combination with MTX in psoriatic arthritis patients who do not respond adequately to MTX alone;
- active ankylosing spondylitis (an arthritis that affects the spine); and
- chronic moderate to severe plaque psoriasis in adult patients (18 years or older) who are candidates for systemic therapy or phototherapy.
For information on drug approvals or to view prescribing information and patient information, please visit Drugs@FDA or DailyMed.

View FDA's Comments on Current Draft Guidance page, for a list of current draft guidances and other topics of interest for patients and caregivers.
|

September 1-2, 2016: FDA participated in a meeting with the European Medicines Agency (EMA) and the Pharmaceuticals and Medical Devices Agency (PMDA, Japan) to discuss regulatory approaches for the evaluation of antibacterial agents.
The EMA, PMDA, and FDA consider that a robust response to the problem of antimicrobial resistance must be multi-faceted and that the regulatory approach for the evaluation of antibacterial agents is only one element of the total response that is required to encourage and accelerate new antibacterial drug development to meet patient needs. View the meeting summary |

In this section you will find a comprehensive list of all the meetings that the FDA is involved with. The meetings may include advisory committee meetings, public workshops and public conferences that are seeking to hear from patients and caregivers.
Most FDA meetings are free to the public and do not require the public to register. Interested persons may present data, information, or views, orally at the meeting, or in writing, on issues pending before the committee. Other types of meetings listed may require prior registration and fees.
|
View FDA's Patient Network Calendar of Public Meetings page for a complete list of meetings and workshops.
For additional information on other agency meetings please visit Meetings, Conferences, & Workshops.
|

Traumatic Brain Injury: FDA Research and ActionsA car accident. A football tackle. An unfortunate fall. These things—and more—can cause head injuries. Head injuries can happen to anyone, at any age, and they can damage the brain.
Here’s how damage can happen: A sudden movement of the head and brain can cause the brain to bounce or twist in the skull, stretching and injuring brain cells and creating chemical changes. This damage is called a traumatic brain injury, or “TBI.”
As kids prepare for school sports, and many adults look ahead to fall activities, the FDA is researching TBI—and encouraging the development of new medical devices to help diagnose and treat it. More information |

FDA Facilitates Research on Earlier Stages of Alzheimer's Disease
Alzheimer’s disease is a nightmare haunting many Americans.
More than 5 million Americans have been diagnosed with the disease, which is the sixth leading cause of death in the United States and the most common cause of dementia among people 60 or older. Alzheimer’s is an irreversible, progressive brain disease that slowly destroys memory and thinking skills. It eventually robs sufferers of the ability to perform even the simplest tasks of daily life.
Despite years of intensive efforts by scientists to develop new safe and effective treatments for Alzheimer’s, options remain limited. In the last 20 years, FDA has approved five drugs for the disease—the most recent one in 2003. Although the drugs can provide some benefit, more needs to be done.
A recent development could bring better results. Three years ago, FDA scientists released a draft guidance that may help companies conduct clinical trials focused on what could be a more treatable stage of the disease: before the onset of noticeable dementia. More information |

Antibacterial Soap? You Can Skip It -- Use Plain Soap and Water
When you buy soaps and body washes, do you reach for products labeled “antibacterial” hoping they’ll keep your family safer? Do you think those products will lower your risk of getting sick, spreading germs or being infected?
According to the FDA, there isn’t enough science to show that over-the-counter (OTC) antibacterial soaps are better at preventing illness than washing with plain soap and water. To date, the benefits of using antibacterial hand soap haven’t been proven. In addition, the wide use of these products over a long time has raised the question of potential negative effects on your health.More information
|

5 Tips for Using Your Microwave Oven SafelyDid you know the FDA regulates microwave ovens? Microwave oven manufacturers must certify their products meet safety performance standards created and enforced by the FDA to protect the public health.
Microwave ovens are generally safe when used correctly. But people have experienced burns, and in rare cases, other injuries from microwave radiation, particularly in cases involving improper use or maintenance. Therefore, always use your oven properly (read on for tips) and maintain it as recommended by the user manual. More information
|
More Consumer Updates
For previously published Consumer Update articles that are timely and easy-to-read and cover all FDA activities and regulated products. More information
For previously published Consumer Update articles that are timely and easy-to-read and cover all FDA activities and regulated products. More information
En Español
La información en esta página es para el público en general, y para profesionales y educadores de salud. Esta información puede ser distribuida y publicada sin previa autorización. En Español
La información en esta página es para el público en general, y para profesionales y educadores de salud. Esta información puede ser distribuida y publicada sin previa autorización. En Español

FDA Cooperative Agreements with States to Advance Food Safety By Stephen Ostroff, M.D., FDA’s Deputy Commissioner for Foods and Veterinary MedicineThe FDA is honoring an important commitment by awarding millions of dollars to the states that will help implement the new produce safety rule mandated by the FDA Food Safety Modernization Act (FSMA) to protect consumers from foodborne illness.
In FSMA, Congress envisioned a strong partnership between the FDA and the state agencies that have a greater understanding of the growing and harvesting practices in their areas. Many have longstanding relationships with farmers and produce associations.
To support this partnership, the FDA is awarding $21.8 million in cooperative agreements to 42 states in support of their work to help implement the new produce rule. These funds will provide states with the resources they need to develop a produce safety system, considering the specific and unique needs of their growers for education, outreach, and technical assistance. To read the rest of this post see FDAVoice, September 9, 2016 |

The Safety Reporting Portal
The Safety Reporting Portal (SRP) streamlines the process of reporting product issues to the Food and Drug Administration and the National Institutes of Health. Whatever your role, (manufacturer, health care professional, researcher, public health official, or concerned citizen), when you submit a safety report through this Portal, you make a vital contribution to the safety of America's food supply, medicines, and other products that touch us all. More information
Center for Food Safety and Applied Nutrition
The Center for Food Safety and Applied Nutrition, known as CFSAN, carries out the mission of FDA. The Center provides services to consumers, domestic and foreign industry and other outside groups regarding field programs; agency administrative tasks; scientific analysis and support; and policy, planning and handling of critical issues related to food and cosmetics. More information
Food Facts for You
The Center for Food Safety and Applied Nutrition, known as CFSAN, issues food facts for consumers to keep you and your family safe. More information |

Hypothyroidism in Dogs: New FDA Treatment Available
Hypothyroidism occurs when the thyroid gland doesn’t produce and secrete enough thyroid hormones. The thyroid gland is located in the mid-neck region near the voice box (larynx). In dogs, the thyroid gland is made up of two separate lobes that lie on either side of the windpipe (trachea). Thyroid hormones play a big role in metabolism and affect the function of many parts of the body. Hypothyroidism is the most common hormone imbalance in dogs and is usually caused by inflammation or shrinkage of the thyroid gland. Hypothyroidism is typically seen in middle-aged to older dogs and occurs more commonly in medium to large breed dogs. More information
Animal Health Literacy
Animal Health Literacy means timely information for the benefit of all animals and their humans. With continuous communication and outreach, the Center for Veterinary Medicine (CVM) strives to enhance the public trust, promote safe and effective use of the animal health products we regulate, and share our scientific endeavors. CVM provides reliable, science-based information to promote animal and human health. More information and Publicaciones en Español del FDA
Animal and Veterinary Updates
Animal and veterinary updates provide information to keep your pets healthy and safe. More information |

How to Report a Pet Food Complaint
You can report complaints about a pet food product electronically through the Safety Reporting Portal or you can call your state’s FDA Consumer Complaint Coordinators. Please provide as much information as possible in your complaint, such as exact name of product, type of container, lot number, UPC codes, how the food was stored, and purchase date and exact location where purchased. If possible, please save the original packaging until the pet food has been consumed. The packaging contains IMPORTANT information often needed to identify the variety of pet food, the manufacturing plant, and the production date.More information |

Deeming Rule Is In Effect—Protecting Kids, Informing AdultsWhen the deeming final rule was announced this past May, Department of Health and Human Services Secretary Sylvia Mathews Burwell summed up the rule's promise, saying it would "help us catch up with changes in the marketplace, put into place rules that protect our kids, and give adults information they need to make informed decisions." The rule, which took effect on August 8, gives FDA the authority to regulate e-cigarettes, hookah tobacco, cigars, and other tobacco products that previously went unchecked under federal law. Some of the first provisions to require compliance are those restricting youth access to these newly-regulated products that, in a troubling trend, have surged in popularity among this group. More information
Public Health Education
Tobacco products are harmful, yet widely used, consumer products that are responsible for severe health problems in both users and non-users. These health problems include cancer, lung disease, and heart disease, which often lead to death.
|

HHS Appeals Board Upholds FDA’s Interpretation of How to Count Violations in Tobacco Civil Money Penalty Enforcement Actions
On June 30, the Department of Health and Human Services’ Departmental Appeals Board (DAB) issued a final decision finding in favor of FDA’s Center for Tobacco Products (CTP) in a case brought by CTP against the tobacco retailer Orton Motor Co., d/b/a Orton’s Bagley. The DAB held that it was reasonable and permissible for CTP to count each time the retailer failed to comply with a tobacco regulation as a violation of the Federal Food, Drug, and Cosmetic Act. More information |
Public Education CampaignsWe are investing in a number of public education campaigns, such as Fresh Empire and The Real Cost, to help educate the public – especially youth – about the dangers of regulated tobacco products. Rooted in science, these efforts are directly linked to our authority to regulate the marketing and sales of tobacco products. More information
Youth and TobaccoWe are working to protect the health of America’s children and ultimately reduce the burden of illness and death caused by tobacco use. More information

FDA and Cosmetic Approval
Cosmetic products are regulated by FDA's Center for Food Safety and Applied Nutrition (CFSAN). CFSAN is responsible for assuring that cosmetics are safe and properly labeled. FDA does not approve cosmetics, although we do approve color additives used in cosmetics. It is the responsibility of cosmetic manufacturers to ensure, before marketing their products, that the products are safe when used as directed in their label or under customary conditions of use. More information |

What is a Cosmetic?
The Federal Food, Drug, and Cosmetic Act (FD&C Act) defines cosmetics by their intended use, as "articles intended to be rubbed, poured, sprinkled, or sprayed on, introduced into, or otherwise applied to the human body...for cleansing, beautifying, promoting attractiveness, or altering the appearance" [FD&C Act, sec. 201(i)]. Among the products included in this definition are skin moisturizers, perfumes, lipsticks, fingernail polishes, eye and facial makeup preparations, cleansing shampoos, permanent waves, hair colors, and deodorants, as well as any substance intended for use as a component of a cosmetic product. More information |
How to Report a Cosmetic Related Problem
You can report a problem you have experienced with a cosmetic to FDA's MedWatch online or by call 1-800-FDA-1088. You can also contact the FDA district office consumer complaint coordinator for your geographic area.
You can report a problem you have experienced with a cosmetic to FDA's MedWatch online or by call 1-800-FDA-1088. You can also contact the FDA district office consumer complaint coordinator for your geographic area.
Recalls and Alerts
To see safety alerts and recent recalls related to cosmetics and other products regulated by FDA. More information
To see safety alerts and recent recalls related to cosmetics and other products regulated by FDA. More information

Information about Expanded Access
Expanded access, sometimes called "compassionate use," is the use outside of a clinical trial of an investigational medical product (i.e., one that has not been approved by FDA). FDA is committed to increasing awareness of and knowledge about its expanded access programs and the procedures for obtaining access to human investigational drugs (including biologics) and medical devices. More information |
Learn about what your physician should do before submitting a request for individual patient expanded access use of an investigational medical product, who may be eligible for expanded access, associated costs, FDA contacts and more. Information for Patients
|
Learn about your responsibilities under the expanded access pathway, how to submit a request for expanded access for an individual patient (including for emergency use), which forms to use, FDA contacts and more. Information for Physicians
|

FDA Patient Network
The FDA Patient Network contains a series of webpages, webinars and presentations on topics related to patient engagement, FDA regulations, understanding medical product (Drugs, Biologics, and Devices) approval and medical product safety updates, Take me to the FDA Patient Network or take me to FDA Webinars.
FDA Basics
Each month, different centers and offices at FDA will host an online session where the public can ask questions to senior FDA officials about a specific topic or just listen in to learn more about FDA. More information
Educational Videos
|

Zika Virus Response Updates from FDA
Zika virus is spread to people primarily through the bite of an infected Aedes species mosquito. Most people never know that they have been infected with the virus. It is estimated that four out of five people with Zika virus infections have no symptoms at all. When symptoms do occur, the most common symptoms are fever, rash, joint pain, and conjunctivitis (red eyes). Even in those who develop symptoms, the illness is usually mild, with symptoms lasting from several days to a week. More information |

healthfinder.gov
Welcome to healthfinder.gov, a government Web site where you will find information and tools to help you and those you care about stay healthy.More information /más información
FDA Email Updates
Sign up for one of the FDA disease specific e-mail list that delivers updates, including product approvals, safety warnings, notices of upcoming meetings, and notices on proposed regulatory guidances. |





















.png)











No hay comentarios:
Publicar un comentario