
01/11/2017
The targeted cancer therapy ibrutinib can effectively treat the symptoms of chronic graft-versus-host disease, a common and serious complication of allogeneic stem cell transplants, findings from a small clinical trial show.
Ibrutinib Relieves Chronic Graft-Versus-Host Disease Symptoms
January 11, 2017, by NCI Staff
The targeted cancer therapy ibrutinib (Imbruvica®) can effectively treat a common and serious complication of a type of stem cell transplant, findings from a small clinical trial show.
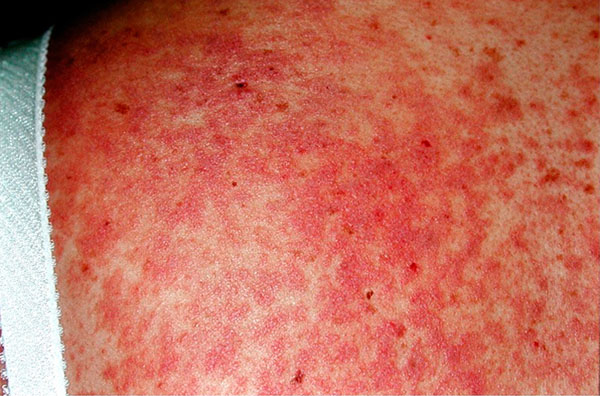
Patients in the trial had blood cancers and were experiencing symptoms of chronic graft-versus-host disease (GVHD) after receiving a transplant of donated blood stem cells, known as an allogeneic transplant. The patients’ symptoms—including widespread skin rashes and painful mouth ulcers—had persisted despite standard treatment with corticosteroids.
Following treatment with ibrutinib, approximately two-thirds of patients in the trial experienced improvements in their GVHD-related symptoms. In 21% of these patients, the symptoms resolved entirely. And the symptom relief was enduring, lasting for up to 5 months or longer in many patients, according to results reported in December at the American Society of Hematology annual meeting.
Having a new option for patients with chronic GVHD who do not adequately respond to corticosteroids would be an important advance, said the trial’s lead investigator, David Miklos, M.D., of the Stanford University Cancer Center.
“Ibrutinib appears to exceed the therapeutic benefits of other agents” for patients experiencing sustained symptoms from GVHD, Dr. Miklos said in a news release.
Steven Pavletic, M.D., of the Experimental Transplantation and Immunology Branch in NCI’s Center for Cancer Research (CCR) agreed.
“This is good news and major progress against chronic GVHD,” said Dr. Pavletic, an expert on the condition. More effective and safer treatments for GVHD are “an area of unmet need,” he continued. “Currently there is no FDA-approved agent for chronic GVHD.”
Chronic GVHD: A Serious Problem
In the United States each year, approximately 10,000 people with blood cancer receive allogeneic stem cell transplants. GVHD occurs when these transplanted cells attack the patient’s healthy cells and tissues. This cellular assault develops in as many as 40% of patients and can cause multiple, often debilitating symptoms, which can also include significant shortness of breath and limb and joint pain.
In addition to these symptoms, GVHD “also has significant mortality associated with it,” explained Wyndham Wilson, M.D., Ph.D., of the CCR Lymphoid Malignancies Branch.
In some patients, GVHD occurs in the first weeks and months after the stem cell transplant, known as acute GVHD. Chronic GVHD occurs when the condition arises or persists months after the transplant.
Corticosteroids are the standard treatment for both acute and chronic GVHD, but for many patients they are not always effective or they eventually stop working, Dr. Wilson said. Long-term use of corticosteroids also can have its own serious side effects, including immune system suppression, he added.
Ibrutinib is approved by the Food and Drug Administration (FDA) for the treatment of some patients with B cell-related cancers, including some types of leukemia and lymphoma. The drug targets a protein known as BTK, which is present in B cells and other types of immune cells. BTK is a component of an important signaling pathway in B cells and blocking it can stop these cells from becoming activated. Ibrutinib also inhibits the activity of a similar protein, ITK, on B cells and T cells. Several studies have shown that improper activation of both of these types of immune cells are common in chronic GVHD.
A study published in 2014 showed that treatment with ibrutinib alleviated symptoms in mouse models of corticosteroid-resistant GVHD. These findings set the stage for human trials of ibrutinib in patients experiencing GVHD.
‘Favorable’ Findings
To be eligible for the phase II trial, patients had to have either a skin rash on more than 25% of their body or significant mouth ulcers despite having received up to three prior regimens for GVHD. The 42 patients in the trial had been experiencing GVHD-related symptoms for many months, with a median duration of 13.7 months.
Treatment with ibrutinib not only led to substantial reductions in GVHD-related symptoms, but many patients in the trial were able to reduce their steroid dose and a handful were able to stop using them altogether.
Using a standardized scoring system for assessing illness-related symptoms, most patients reported having far less severe symptoms than before they started ibrutinib. And many patients who responded to the drug had reductions in the levels of blood-based markers associated with GVHD, including those linked to inflammation and connective tissue scarring.
Nearly half of patients in the trial experienced serious side effects from ibrutinib, including high fevers and pneumonia. These side effects are typical for patients treated with ibrutinib, said Samantha Jaglowski, M.D., an investigator on the trial from the Ohio State University James Cancer Center.
Overall, Dr. Jaglowski continued, most of the side effects were manageable.
For many patients with chronic GVHD, Dr. Wilson said, the risk of side effects from ibrutinib may well outweigh the impact of the GVHD symptoms they’re experiencing. He called the trial results “remarkable,” given how long patients in the trial had been experiencing GVHD-related symptoms.
“These are people who have already gone through the standard therapies and clearly their symptoms were being poorly controlled,” he said. “Chronic GVHD can be a terrible condition, and these results are extremely favorable.”
Moving Toward the Clinic
It’s still too early to say what kind of impact these results will have on the care of patients with chronic GVHD, Dr. Jaglowski said.
Earlier this year, the FDA granted ibrutinib a Breakthrough Therapy Designation for patients with chronic GVHD that is not being controlled by other therapies like corticosteroids. This designation allows the FDA to expedite its review of a drug for marketing approval based on early data suggesting that it has efficacy in a serious or life-threatening disease or condition.
One important question that needs to be answered is how long patients with chronic GVHD need to take ibrutinib to control their symptoms, Dr. Wilson said.
When it comes to drugs that are involved in suppressing an immune-related attack, he explained, “sometimes it’s just a matter of breaking the cycle.” Patients who respond well to ibrutinib could go on a “drug holiday,” he continued, to see if the symptom relief persists.
“This is clearly a first study and as more experience is gained with using ibrutinib in this patient population, we will learn better how to use it,” Dr. Pavletic said.
More information on the other drugs patients in the trial were using to help control GVHD-related symptoms or infections will be important moving forward, he continued, as some may interact with ibrutinib and lead to or worsen side effects. He noted that 14 patients in the trial had to drop out because of side effects.
“I anticipate that, with more experience and attention to possible drug interactions and ibrutinib dose adjustments, the rate of side effects may actually go down,” he continued. “It is very encouraging that 12 patients maintained sustained responses for up to 2 years, and my hope is that more treated patients could get to that point.”
At least one trial may help to address some of the outstanding questions around using ibrutinib in patients with GVHD.
AbbVie and Pharmacyclics, which jointly market ibrutinib, plan to launch a phase III clinical trial of ibrutinib to treat GVHD in the first half of 2017. However, the trial will test the drug as part of initial therapy for patients with chronic GVHD, not patients who have already developed treatment resistance.
According to a spokesperson for Pharmacyclics, the companies will file for regulatory approval of ibrutinib for the treatment of chronic GVHD later this year.























.png)











No hay comentarios:
Publicar un comentario