

New Eczema Drug Promising in Early Trial
Nemolizumab significantly reduced the itch and improved appearance of skinThursday, March 2, 2017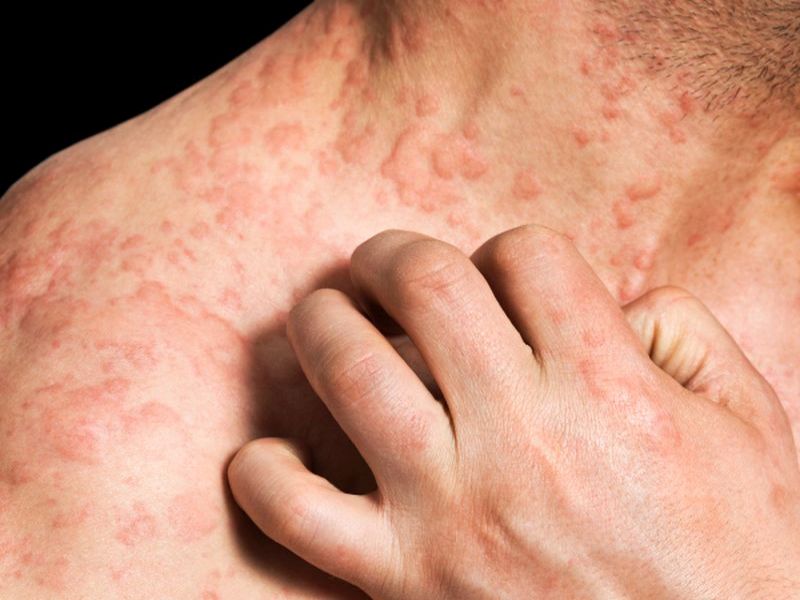

THURSDAY, March 2, 2017 (HealthDay News) -- An experimental drug may significantly reduce the itching and improve the appearance of moderate to severe eczema, a new, preliminary trial finds.
Nemolizumab is a man-made, injectable antibody that acts against the protein that has been identified as playing a part in eczema, the international team of researchers said.
"The treatments for atopic dermatitis [eczema] have been disappointing because of their lack of efficacy and the long-term side effects," said Dr. Doris Day, a dermatologist at Lenox Hill Hospital in New York City. She had no role in the study.
"There are also issues with compliance, since the products often need to be applied to broad areas multiple times a day," she added.
Since this is a chronic condition, continued treatment is usually needed to maintain results, Day explained.
"The goal is to find a non-steroid treatment that is easy to follow, and with reliable results and minimal adverse effects," she said.
While the hope is always for a cure, the results of this trial "are encouraging and give hope to those suffering from moderate to severe atopic dermatitis [eczema] for an effective treatment to control their condition with good long-term outcomes," Day said.
The study was published March 2 in the New England Journal of Medicine and was funded by Tokyo-based Chugai Pharmaceutical Co. Ltd., the maker of nemolizumab.
Most types of eczema cause dry, itchy skin and rashes on the face, inside the elbows, behind the knees, and on the hands and feet. Scratching can cause the rash to turn red, swell and itch even more, according to the U.S. National Institutes of Health.
Eczema is not contagious. Its cause is not known, but is likely due to both genetic and environmental factors. It may get better or worse over time, but it is often a long-lasting disease.
In this 12-week trial, a team lead by Dr. Thomas Ruzicka, from the department of dermatology and allergology at Ludwig Maximilian University in Munich, Germany, randomly assigned 264 patients with moderate to severe eczema to one of three injectable doses of nemolizumab or placebo.
The researchers found those who received nemolizumab every four weeks had significant improvement in their eczema, compared with patients who received a placebo shot.
Among the 216 patients who completed the study, those getting the second-highest dose of nemolizumab, which the researchers considered to have the best effect with the lowest risk, experienced a 60 percent reduction in itching, compared with a 21 percent reduction among patients who received a placebo.
In addition, patients receiving the second-highest dose of the drug saw a 42 percent reduction in the size of the areas of eczema, compared with a 27 percent reduction among those receiving a placebo.
Patients getting that dose also had a 20 percent reduction in the total body area affected by eczema, compared with a less than 16 percent reduction among those receiving placebo, the researchers found.
One dermatologist was impressed with the findings.
"The positive results of the phase 2 clinical trial is exciting news for those of us who take care of such patients and, of course, for the patients themselves," said Dr. Robert Skrokov, an attending dermatologist at Northwell Health's Southside Hospital in Bay Shore, N.Y.
Until now, patients who fail to respond to intensive topical therapy or phototherapy have had to be treated with drugs that severely suppress their immune systems, he explained.
"We anxiously await the results of phase 3 trials on nemolizumab," Skrokov said. "Hopefully, we can achieve the same kind of safety and efficacy demonstrated by the biologics which have made such a dramatic difference in the lives of patients with severe psoriasis and psoriatic arthritis."
In the phase 2 trial, 17 percent of patients did withdraw because of side effects, which included worsening eczema, respiratory tract infections, infections of the nose or throat, or swelling of the ankles or feet.
SOURCES: Doris Day, M.D., dermatologist, Lenox Hill Hospital, New York City; Robert Skrokov, M.D., attending dermatologist, Northwell Health's Southside Hospital, Bay Shore, N.Y.; March 2, 2017, New England Journal of Medicine
HealthDay
Copyright (c) 2017 HealthDay. All rights reserved.
News stories are written and provided by HealthDay and do not reflect federal policy, the views of MedlinePlus, the National Library of Medicine, the National Institutes of Health, or the U.S. Department of Health and Human Services.
- More Health News on:
- Eczema








































No hay comentarios:
Publicar un comentario