
Huntington’s Disease: Gene Editing Shows Promise in Mouse Studies
 My father was a folk song collector, and I grew up listening to the music of Woody Guthrie. On July 14th, folk music enthusiasts will be celebrating the 105th anniversary of Guthrie’s birth in his hometown of Okemah, OK. Besides being renowned for writing “This Land is Your Land” and other folk classics, Guthrie has another more tragic claim to fame: he provided the world with a glimpse at the devastation caused by a rare, inherited neurological disorder called Huntington’s disease.
My father was a folk song collector, and I grew up listening to the music of Woody Guthrie. On July 14th, folk music enthusiasts will be celebrating the 105th anniversary of Guthrie’s birth in his hometown of Okemah, OK. Besides being renowned for writing “This Land is Your Land” and other folk classics, Guthrie has another more tragic claim to fame: he provided the world with a glimpse at the devastation caused by a rare, inherited neurological disorder called Huntington’s disease.When Guthrie died from complications of Huntington’s a half-century ago, the disease was untreatable. Sadly, it still is. But years of basic science advances, combined with the promise of innovative gene editing systems such as CRISPR/Cas9, are providing renewed hope that we will someday be able to treat or even cure Huntington’s disease, along with many other inherited disorders.
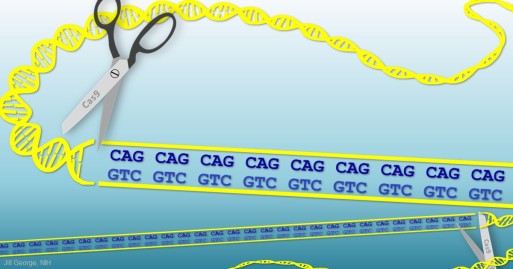
My own lab was part of a collaboration of seven groups that identified the Huntington’s disease gene back in 1993. Huntington’s disease occurs when a person inherits from one parent a mutant copy of the huntingtin (HTT) gene that contains extra repetitions, or a “stutter,” of three letters (CAG) in DNA’s four-letter code. This stutter leads to production of a misfolded protein that is toxic to the brain’s neurons, triggering a degenerative process that, over time, leads to mood swings, slurred speech, uncontrolled movements, and, eventually, death. In a new study involving a mouse model of Huntington’s disease, researchers were able to stop the production of the abnormal protein by using CRISPR tools to cut the stutter out of the mutant gene.
The progress, reported in the Journal of Clinical Investigation [1], comes from the NIH-supported team of Su Yang, Renbao Chang, Xiao-Jiang Li, and colleagues at Emory University School of Medicine, Atlanta. The group’s previous work showed that halting the production of mutated (or even healthy!) HTT protein in mature neurons doesn’t hurt the cells or cause obvious neurological problems in mice [2]. So, the researchers now wanted to see if halting HTT production in millions of neurons in the striatum, which is a part of the inner brain that controls motor skills, could reverse early signs of disease that typically appear in affected mice before the age of 9 months.
To get their answers, the researchers injected millions of inactivated viral particles directly into the striatum of a few 9-month-old mice, engineered to produce the mutant form of HTT protein. Each particle, like a Trojan horse, delivered to the neurons one of the two pieces of the CRISPR/Cas9 editing system: either a short guide RNA sequence to mark for removal the HTT gene’s CAG repeats or a scissor-like Cas9 enzyme to snip out the repeats. In this strategy, both the health and abnormal copies of the HTT gene were “knocked out,” resulting in the production of no HTT protein.
Remarkably, three weeks later, the researchers found that the CRISPR/Cas9 gene editing had reversed the disease process in their mouse model. Neurons in the striatum had stopped making the HTT protein. What’s more, the toxic, abnormal HTT protein that had already clumped together in and around the neurons—and which likely would have would have killed them—had begun to clear to varying degrees in the mice. The same went for other protein abnormalities associated with the progression of Huntington’s disease.
There was even better news to come. The Emory team repeated the CRISPR/Cas9 injections into the striatum of a dozen 9-month-old mice and got a similar protein-clearing outcome. Then, over the next three months, the researchers found that the animals’ balance, muscle coordination, and mobility had improved compared to mice given sham shots of CRISPR/Cas9. Interestingly, the degree of improvement in their motor skills corresponded with the amount of toxic protein that had been cleared from the striatum.
As exciting as gene editing is as a potential treatment for Huntington’s disease, the research is still very much in its early stages. For example, while the Emory researchers were able to establish that adult mice could live well without a functioning copy of HTT, they remain uncertain whether that’s also the case in humans.
Another potential safety concern with CRISPR/Cas9 is off-target editing. Last May, in a very controversial article, it was reported that CRISPR/Cas9 can sometimes go astray and snip away at healthy genes in animal studies, leaving behind hundreds of unintended mutations in its wake [3]. However, the Emory team reported that off-target editing did not appear to be a major problem in its latest study. Sequencing of genomic DNA taken from the striatum of the mice showed that CRISPR/Cas9 editing occurred “predominantly” around their target sequences without significant genomic editing in the most likely off-target locations. While this is only one study, it’s reassuring news as more animal studies testing the potential curative power of CRISPR/Cas9 editing move forward.
This utilization of CRISPR/Cas9 to pursue a cure for Huntington’s disease is one more example of how this powerful new technology might be applied to the thousands of diseases due to a specific mutation in DNA; efforts are already underway for other conditions like sickle cell disease and muscular dystrophy. Given the promise, the NIH Common Fund is actively exploring ways in which this approach could be accelerated.
References:
[1] CRISPR/Cas9-mediated gene editing ameliorates neurotoxicity in mouse model of Huntington’s disease. Yang S, Chang R, Yang H, Zhao T, Hong Y, Kong HE, Sun X, Qin Z, Jin P, Li S, Li XJ. J Clin Invest. 2017 Jun 19. [Epub ahead of print]
[2] Ablation of huntingtin in adult neurons is nondeleterious but its depletion in young mice causes acute pancreatitis. Wang G, Liu X, Gaertig MA, Li S, Li XJ. Proc Natl Acad Sci U S A. 2016 Mar 22;113(12):3359-3364.
[3] Unexpected mutations after CRISPR-Cas9 editing in vivo. Schaefer KA, Wu WH, Colgan DF, Tsang SH, Bassuk AG, Mahajan VB. Nat Methods. 2017 May 30;14(6):547-548.
Links:
Huntington’s Disease Information Page (National Institute of Neurological Disorders and Stroke/NIH)
Li Laboratory (Emory University, Atlanta)
NIH Support: National Institute of Neurological Disorders and Stroke






















.png)











No hay comentarios:
Publicar un comentario