
By: Pamela E. Scott, Ph.D.
When women are pregnant they take care to eat right and refrain from smoking and drinking alcoholic beverages. But what to do about prescription drugs is a more complicated topic.
There are very few prescription medications that have been specifically approved for use during pregnancy. And yet, doctors in clinical practice must prescribe needed medicines to pregnant women to treat a variety of illnesses and conditions such as diabetes, high blood pressure or even something as simple as a dental infection.
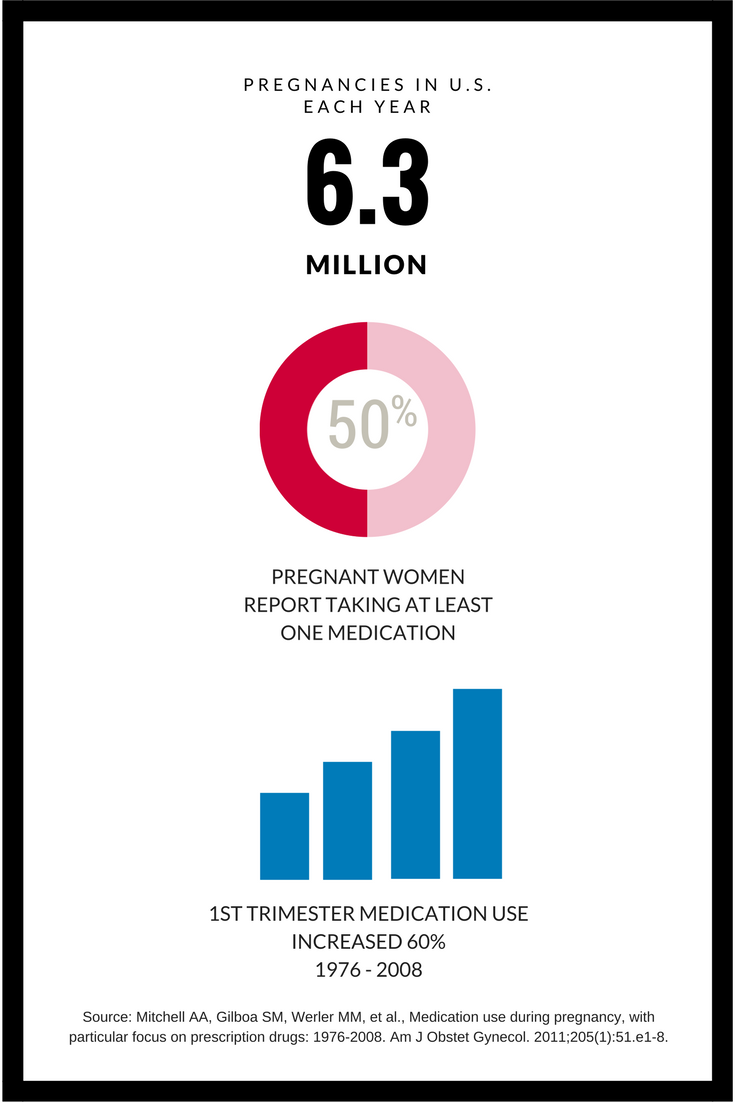
Indeed, about half of the 6.3 million women who are pregnant every year take at least one medication, and prescription use is on the rise, up by more than 60 percent from 1976 through 2008.
More information is clearly needed. The 21st Century Cures Act, which was enacted in 2016, established a task force to consider what is being done to identify and address gaps in knowledge and research on safe and effective therapies for pregnant and lactating women. Within 18 months after it is established, the task force will develop a report to Congress with specific recommendations for addressing the issues identified. The Office of Women’s Health (OWH) is leading FDA’s activities for the task force. My colleagues at OWH will be working with FDA’s Centers to promote dialogue and research collaboration. We look forward to hearing from our public and private partners at the Task Force's two-day public meeting, which begins today. Continue reading
Indeed, about half of the 6.3 million women who are pregnant every year take at least one medication, and prescription use is on the rise, up by more than 60 percent from 1976 through 2008.
More information is clearly needed. The 21st Century Cures Act, which was enacted in 2016, established a task force to consider what is being done to identify and address gaps in knowledge and research on safe and effective therapies for pregnant and lactating women. Within 18 months after it is established, the task force will develop a report to Congress with specific recommendations for addressing the issues identified. The Office of Women’s Health (OWH) is leading FDA’s activities for the task force. My colleagues at OWH will be working with FDA’s Centers to promote dialogue and research collaboration. We look forward to hearing from our public and private partners at the Task Force's two-day public meeting, which begins today. Continue reading





















.png)












No hay comentarios:
Publicar un comentario