| New from NCI |
| Finding Health Care Services |
 | | This page has been expanded to include information from the fact sheet “How to Find a Doctor or Treatment Facility if You Have Cancer,” which has been removed from the NCI website. |
| Risk of Heart Attack and Stroke May Be Higher after a Cancer Diagnosis |
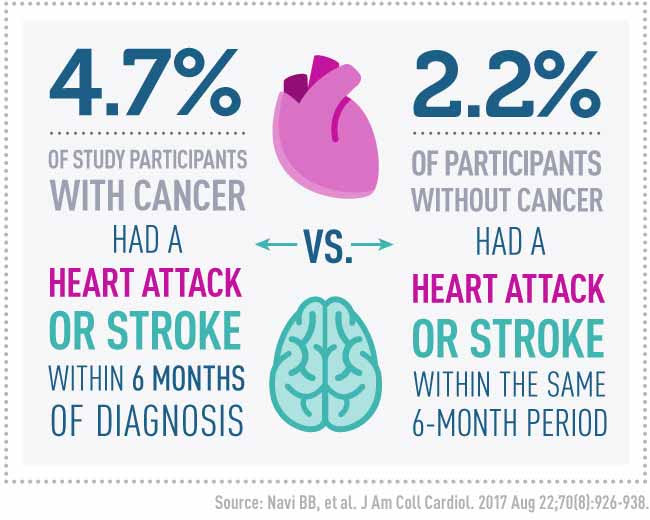 | | A new study suggests that a diagnosis of cancer can come with an increased risk of a heart attack or stroke in the months after being diagnosed. |
| Food and Drug Administration (FDA) Approves Immunotherapy Drug for Some Metastatic Colorectal Cancers |
 | | FDA has granted accelerated approval to nivolumab (Opdivo®) for patients with metastatic colorectal cancer whose tumors have genetic changes that affect DNA repair. |
Test Shows Promise for Early Detection of Pancreatic Cancer
Early research suggests that a new blood test may be able to find pancreatic cancer in its earliest stages, when it is most likely to respond to treatment. |
Targeted Therapy Approved for HER2-Positive Breast Cancer
FDA has approved neratinib (Nerlynx™) for patients with early-stage HER2-positive breast cancer who have finished at least 1 year of adjuvant therapy with trastuzumab.
|
| Drug Information Updates |
New Approval for Certain Colorectal Cancers
We’ve updated our nivolumab drug information summary to include a recent approval by FDA. Nivolumab is now approved to treat colorectal cancer with certain genetic features: mismatch repair deficiency and high microsatellite instability. It is used in adults and children 12 years and older. |
New Drug for Leukemia
We’ve added a new drug information summary for enasidenib mesylate, which was recently approved by FDA to treat recurrent or refractory acute myeloid leukemia (AML) that has a certain genetic mutation. |









No hay comentarios:
Publicar un comentario