
“Selfish” Gene Enhances Own Transmission at Expense of Organism’s Fertility
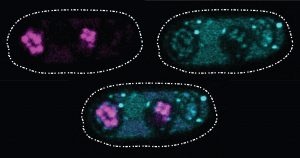
These glowing images of yeast (Schizosaccharomyces kambucha) reproductive cells show an example of a selfish gene at work. Here, the selfish gene boosts its chances of being passed to the next generation by producing both a toxin (stained cyan) and an antitoxin (stained magenta). Cells with a copy of the selfish gene are protected by the antitoxin, left and bottom ovals. Those without the selfish gene are destroyed by the toxin. Scientists suspect that selfish genes could be operating throughout many organisms’ genomes, possibly having a major impact on how genetic material is inherited over generations. Credit: Image courtesy of María Angélica Bravo Núñez and Nicole Nuckolls.
There’s an old saying that rules are meant to be broken. In the 1860s, Gregor Mendel found that each copy of a gene in an organism has an equal chance of being passed to the next generation. According to this simple rule, each version of a gene gets passed to offspring with the same frequency. The natural selection process can then operate efficiently, favoring the genes that produce an advantage for an organism’s survival or reproductive success and, over successive generations, eliminating genes from the gene pool that bring a disadvantage.
Of course, the way organisms inherit genes is not as straightforward as Mendel’s work predicted. In natural systems, inheritance often fails to follow the rules. One culprit scientists are identifying again and again are what are called “selfish genes”: one or more genes that have evolved a method of skewing inheritance in their favor.
Scientists refer to these selfish genes—which are often complexes of multiple genes working together—as “selfish” because they enhance their own transmission to the next generation, sometimes by killing off any of the organism’s reproductive cells that lack copies of those genes. Using a variety of techniques, the genes are effective at passing themselves on to future generations. However, their methods set up a conflict within the organism by damaging its fertility; overall, fewer reproductive cells or offspring survive to produce a new generation.
Selfish genes are a challenge for scientists to identify, but researchers say that knowing more about these genes could help explain a range of genetic mysteries, from causes of infertility to details on how species evolve. The methods these genes use could also be harnessed to help control the reproduction of certain populations such as mosquitos that spread disease.
One recently described selfish gene system is found in the yeast cells pictured above. Sarah Zanders  and her colleagues at the Stowers Institute for Medical Research in Kansas City, Missouri, and the Fred Hutchinson Cancer Research Center in Seattle, Washington, study selfish gene systems in yeast to understand how common they are and how they affect a species’ fertility and evolution. “Usually we think about infertility stemming from the good guys failing. For example, a gene that normally promotes fertility could be mutated so that it can no longer do its job,” says Zanders. “But selfish genes are another potential source of infertility. Learning general principles about selfish genes in simple models will guide future searches for selfish genes that could be contributing to human infertility.”
and her colleagues at the Stowers Institute for Medical Research in Kansas City, Missouri, and the Fred Hutchinson Cancer Research Center in Seattle, Washington, study selfish gene systems in yeast to understand how common they are and how they affect a species’ fertility and evolution. “Usually we think about infertility stemming from the good guys failing. For example, a gene that normally promotes fertility could be mutated so that it can no longer do its job,” says Zanders. “But selfish genes are another potential source of infertility. Learning general principles about selfish genes in simple models will guide future searches for selfish genes that could be contributing to human infertility.”
Recently, the scientists discovered a single selfish gene, wtf4, that encodes both a toxin and an antitoxin protein. When yeast produce their reproductive cells, called spores, the wtf4 toxin protein is released into the immediate vicinity, but the antitoxin remains inside spores that contain a copy of wtf4. The toxin destroys all the spores that don’t have the antitoxin protein. Although the yeast has fewer spores—and therefore reduced fertility—each spore carries wtf4, ensuring that the gene will be passed to the next generation of yeast.
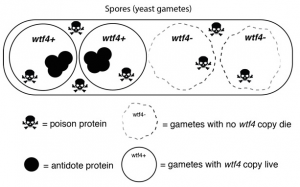
Yeast reproductive or gamete cells (called spores) show how selfish genes can influence inheritance. Spores that don’t carry the wtf4 gene are killed when the wtf4 poison protein is released into the area around them. Those with a wtf4 copy (the selfish gene) are protected against the toxin by the antidote protein. Credit: Sarah Zanders laboratory, Stowers Institute for Medical Research.
Researchers have found that selfish gene systems such as wtf4 crop up in many other species. They use a variety of mechanisms to ensure that they survive from generation to generation. In bacteria, some selfish genes can even be beneficial to their hosts. For example, some bacteria have figured out a way to incorporate into their genomes selfish gene systems to respond to stresses such as infection, starvation, or treatment with antibiotics. The selfish elements include toxins that are chemically bound to antitoxins. When the bacterium is exposed to stress, it produces enzymes that cut the bond between the toxin and antitoxin. The enzymes also degrade the antitoxin. The released toxin then stops the bacterium from making other proteins, which sends it into a dormant, or hibernation-like, state until conditions improve.
Selfish genes, scientists are beginning to realize, influence many aspects of inheritance. For example, genes located near a selfish gene in an organism’s genome may be passed to the next generation simply because of proximity, even if they are damaged or produce traits that are not helpful to the organism. For example, researchers found that a selfish gene system in fruit flies causes a large piece of DNA to be passed along unchanged, even though that piece causes sterility in female flies, which inherit two copies of this genetic material. This “selfish-by-association” mechanism may help to explain how a genetic disorder persists in a population.
Sometimes the presence of selfish genes masks how genes actually function. Leonid Kruglyak  and his colleagues at the University of California, Los Angeles (UCLA), recently described a selfish element in nematode worms (Caenorhabditis elegans) that causes 25 percent of embryos to die when certain strains of the worms are mated. Researchers had thought a certain gene, pha-1, was an essential regulator of development, because mutant worms lacking a working copy of pha-1 would fail to develop the feeding organ, or pharynx. In fact, the pha-1 protein only suppressed the effects of a toxin, sup-35. This selfish gene system, the scientists found, hijacks a developmental pathway to kill the embryos that do not inherit sup-35/pha-1.
and his colleagues at the University of California, Los Angeles (UCLA), recently described a selfish element in nematode worms (Caenorhabditis elegans) that causes 25 percent of embryos to die when certain strains of the worms are mated. Researchers had thought a certain gene, pha-1, was an essential regulator of development, because mutant worms lacking a working copy of pha-1 would fail to develop the feeding organ, or pharynx. In fact, the pha-1 protein only suppressed the effects of a toxin, sup-35. This selfish gene system, the scientists found, hijacks a developmental pathway to kill the embryos that do not inherit sup-35/pha-1.

Caenorhabditis elegans. Credit, iStock.
The scientists believe that other genes thought to be essential to development may turn out to be elements of selfish gene systems. Eyal Ben-David and Alejandro Burga, lead authors of Kruglyak’s study, said that their finding points out the importance of studying natural genetic variation in a species. “Most researchers use a single genetic background [that is, they study organisms that all have exactly the same genetic code] because it increases reproducibility,” Ben-David says. “You will get most things right, but you will also miss many important characteristics that make individual organisms and populations unique. In our case, by studying C. elegans from diverse locations in the world, we were able to identify wild worms that lacked pha-1, which helped us solve the puzzle.”
Because selfish genes reduce fertility, any species that finds a way to suppress these genetic elements would have a fertility advantage, because more of its offspring would survive. Indeed, organisms do appear to evolve the ability to suppress their selfish genes. Scientists believe that selfish genes must themselves change rapidly across generations to evade such suppressors, setting off a kind of arms race of evolution between selfish genes and the organism’s adaptations to stop them. For this reason, organisms may have many, very diverse families of selfish genes, making it hard for scientists to identify them.
Knowing how these genes sabotage reproduction is important. For example, selfish gene systems could offer a means for controlling or minimizing the harm caused by disease-carrying or crop-destroying insects. Insects could be developed that carry the selfish genes, which, in turn, would reduce the number of insects produced over time. However, many scientists warn that because so little is known about how selfish genes interact with the natural environment, it is impossible to project the full consequences of releasing these altered insects into wild populations. According to Ben-David and Burga, the effects that selfish elements have on inheritance may help drive the formation of new species among the plants or animals that they affect. Gaining a better understanding of selfish genes will enable scientists to avoid unanticipated consequences of using selfish genes as a tool—for the organism, for its future generations, and for our environment.
Zanders’s research on yeast was funded in part by NIGMS under Grants R01GM031693,R35GM118120 , R01GM74108 , and R00GM114436 . Kruglyak’s research on nematodes was funded in part by the NIH under Grant R01HG004321.





















.png)












No hay comentarios:
Publicar un comentario