
Free Radical Formation in Electronic Cigarette Aerosols
 Thought LeadersDr John RichieProfessor of Public Health Sciences
Thought LeadersDr John RichieProfessor of Public Health Sciences& PharmacologyPenn State College of Medicine
An interview with Dr John Richie, conducted by Stuart Milne, BA.
Your presentation at Pittcon focused on free radical formation in electronic cigarette (EC) aerosols. What have been the main challenges associated with characterizing the harmful by-products EC can produce?
Firstly, one of the main challenges is the fact that they're so new and they've exploded onto the market. Secondly, and perhaps most importantly, is the fact that there are so many different types of products both in terms of the e-cigarettes themselves and the liquids that you fill them with.
 © Tibanna79/Shutterstock.com
© Tibanna79/Shutterstock.comThere are literally thousands of different products and styles on the market and they're changing rapidly. This rapid introduction of e-cigarettes and the variety available makes it a difficult challenge to cover them all. Our research has already shown that the results are not representative across the various varieties available on the market.
Why are free radicals bad in the context of electronic cigarettes?
Free radicals are found in a variety of places as you might imagine. They're found in very high concentrations in cigarettes smoke where, as oxidants, they are considered extremely toxic. They're also thought to be an influencing factor in many diseases, if not all the diseases caused by tobacco smoke. Therefore, it makes sense that we look for them in e-cigarettes. The fact that we find them in pretty high quantities is a little bit troubling in terms of the potential harm that might come from e-cigarette exposure.
Are these free radicals consistent across all electronic cigarettes or does it depend on the type, temperature or e-liquids used?
What's consistent is that we find free radicals in every combination of product and e-liquid we've tested. However, the levels of these radicals can vary by the type of e-cigarette product, with higher levels being produced in the more powerful designs. The products available on the market today have vastly different levels of power. Some are stronger than others.
The free radicals produced can also vary by how these e-cigarettes are used. For example, the size of the puffs, the length of the puffs and the intensity of these puffs all influence the free radicals produced. They can also vary by flavor additives. Some additives are associated with increased levels of radicals, whereas others may be associated with decreased levels of radicals.
A paper you recently co-authored suggested that free radical production increased in a temperature-dependent manner. Please could you elaborate on your findings and the influencing factors behind these increases?
Temperature's a very important factor in e-cigarettes because it’s related to the power of these e-cigarettes. The higher the temperature, the more aerosol produced, the more vapor you get, and the more nicotine you get. Our research has shown that these products can vary quite dramatically in terms of the temperatures. Some operate at relatively lower temperatures around 200°C. Some operate upwards of 300°C. It's very important for us to know whether the different usage conditions can impact the toxicants that we're being exposed to.
In the case of free radicals, we did find that the higher the temperature, the higher the number of radicals that are being produced. When we looked at the e-cigarettes operating upward of 300°C the level of radicals increased almost exponentially.
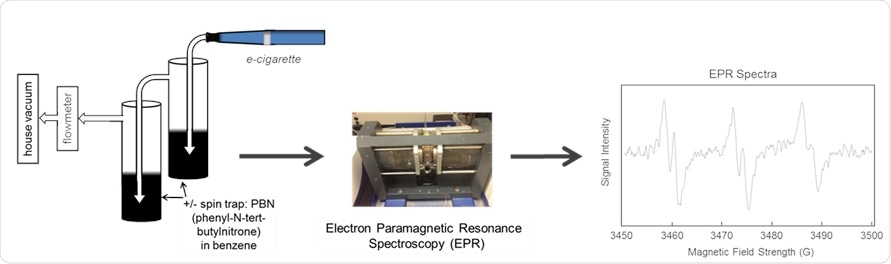
Production and measurement of free radicals in e-cigarette aerosols.
Flavoring chemicals are a common component in e-liquids. Was there any difference in free radical production based on the flavouring chemicals used?
Flavors seem to have a pretty large impact on the radicals that are produced. There are over 7,000 different flavors available on the market today. Just to make things even more complex, each of these flavorants are made up of one or many flavoring chemicals. These chemicals each have their own different properties and they can interact with one another. It's a very complex landscape.
We've been able to test between 50 and 60 different flavors so far in terms of their impacts on free radical production. We find that many of these flavors are associated with enhanced or higher levels of free radical production. However, there are some that are associated with lower levels of free radical production. We think that might have something to do with some anti-oxidant properties of some flavors.
What characterization methods do you use to measure these free radicals?
One of the main problems with measuring these free radicals in e-cigarettes is that they're very short lived, which makes them very hard to measure. They've very reactive, which probably leads to one of the reasons why they're bad for you from a biologic perspective. What we have to do is trap them. We use spin traps, which are chemicals that will trap these free radicals and turn them into longer-lived free radicals. We can measure these longer-lived radicals using an instrument called an electron paramagnetic resonance spectrometer or EPR, which is the only method that you can measure free radicals with directly.
What have your findings suggested and what are the potential impacts for human health?
That’s the big question. We know that these are highly reactive radicals, but we don't know their chemical identity yet. We're working on that and we're close to it, but we can't ascribe specific chemical structures to them at this point in time. That will help in terms of trying to figure out exactly how toxic they may be. We have done some experiments where we have exposed biologically relevant chemicals, lipids, to these free radicals that were produced from e-cigarettes.
We found that these free radicals oxidized these lipids in a predictable manner. We believe that these free radicals are damaging from a biological perspective. We don’t yet know exactly how damaging they are in a real cell.
Can electronic cigarettes be designed to minimize exposure to these potentially harmful products?
Our work is aiming to measure the levels of radical’s produced by e-cigarettes under real conditions. We want to use this data to develop products that don’t produce these toxicants.
In the case of free radicals, for example, we know that there are some flavorants that, when added to the e-liquid, can reduce the levels of free radicals. I think that alone suggests that there may be promise in terms of designing an e-liquid and an e-cigarette device that may produce negligible levels of radicals.
What are the next steps in your research?
The next step is to map out the chemical identity of these radicals. That will allow us to go a long way in terms of predicting how toxic they may be. Secondly, I think it'll be important to understand the toxicity of these free radicals in biological systems. That will involve exposing cells to e-cigarette aerosols and determining the specific effects of these radicals on important cell functions.
Why is Pittcon important for sharing your research?
I think the importance of this group stems from their expertise in measurements. E-cigarettes create a challenge in terms of measuring the radicals that are being produced from them. I think some of the techniques, some of the expertise here at Pittcon can be greatly used in terms of trying to tease apart what these e-cigarettes are producing. I'm hoping that some of the people in the audience at my talk had some neat ideas in terms of trying to see what's happening with these new and ever-changing devices.
About Dr. John Richie
 Dr. Richie is Professor of Public Health Sciences and Pharmacology at Penn State University College of Medicine in Hershey, PA. Dr. Richie’s research over the past 30 years has focused on elucidating mechanistic links between the biological aging process and the development of aging-related diseases and disorders.
Dr. Richie is Professor of Public Health Sciences and Pharmacology at Penn State University College of Medicine in Hershey, PA. Dr. Richie’s research over the past 30 years has focused on elucidating mechanistic links between the biological aging process and the development of aging-related diseases and disorders. Using an interdisciplinary research approach, a major aim has been on the role of oxidative stress, generated both endogenously through normal metabolism and from environmental exposures such as tobacco, as a mechanism for enhanced susceptibility to diseases during aging. In addition, his research has focused on the critical role of glutathione, the body’s major antioxidant and redox regulator, in protecting against and responding to oxidative challenges.
With the ultimate goal of developing novel disease prevention strategies and improved healthspan, Dr. Richie’s lab continues to translate its laboratory findings by testing promising intervention protocols aimed at enhancing cellular protective mechanisms in both preclinical models and clinical trials.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.






















.png)











No hay comentarios:
Publicar un comentario