
qEV Size Exclusion Columns for Fast and Reliable Isolation of Extracellular Vesicles from Plasma
Introduction
The major role of extracellular vesicles, also known as EVs, is to mediate intercellular communication. These phospholipid bilayer membrane vesicles also play a role in the regulation of a wide range of biological processes by transmitting biological information between cells. EVs are composed of proteins, lipids, nucleic acids, and membrane receptors of the cells from which they originate.
There is growing evidence that EVs are significantly involved in normal development, physiological processes, viral infections, and human disease. EVs are present in many biological fluids; they are found in abundance in plasma which can be easily extracted by drawing the blood of a patient.
Plasma is however also a complex biological sample that is rich in high- and low-density lipoproteins (HDL/LDL), protein, nutrients, salts, ions, and other particulates which need to be removed prior to EV analysis.
Chemical precipitation and ultracentrifugation are traditional techniques used for isolating EVs, but these methods produce impure aggregated vesicles and are costly in terms of equipment and time.1,2 Considering these issues, a fast, simple, standardized, and economical method is required to extract EVs from plasma.
qEV size exclusion columns for EV isolation from plasma
Using the qEV Size Exclusion Columns from Izon Science, the EVs can be quickly isolated from plasma. Background proteins, solutes, lipids, cell debris, and other particulates are removed by each column, while the biological properties and integrity of EVs are maintained.
When working with highly enriched EV samples, the accuracy and sensitivity of downstream assays (for example, RNA profiling, protein profiling, etc.) are enhanced. As a result of rapid isolation, which takes a mere 15 minutes, EV samples can be easily collected, prepared for analysis, and then examined by a qNano Gold System – all in less than 1 hour.
Materials and methods
EVs are isolated from plasma in the following way:
- 4 mL of human venous blood is drawn from a patient and transferred to a BD Vacutainer® tube that contains sodium citrate as an anticoagulant.
- After mixing the content, the Vacutainer tube is centrifuged (1,500 x g, 10 minutes) to isolate the plasma from the cells and the plasma supernatant is then transferred to a clean test tube and centrifuged (3,000 x g, 15 minutes). The plasma supernatant is again transferred to a clean test tube and centrifuged once more.
- 550 μL aliquots of platelet free plasma (PFP) supernatant is then transferred to several 1.5 mL microcentrifuge tubes*, leaving a plasma residue above the pellet area. The tubes are set aside.
- The qEV Size Exclusion Column is prepared by removing the luer-slip cap from the column outlet, and the column is flushed with 10 mL particle-free phosphate-buffered saline (PBS) solution that is freshly prepared. Particle-free PBS solution is prepared by dissolving PBS tablets (Sigma part number P4417-100) in Milli-Q®(Millipore Corporation) water and filtering through a 0.22-μm filter. The luer-slip cap is reinstalled onto the column outlet and the PBS solution is finally removed above the top frit.
- 500 μL of PFP is transferred to the qEV Size Exclusion Column, the luer-slip cap is removed from the column outlet, and 500 μL fractions are collected.
- Fractions were checked for both EVs and protein concentration through a qNano Gold System and absorbance at 280nm, respectively. For fractions that contain EVs, Pierce BCA protein assay (Thermo Fisher Scientific, Inc.) was employed to determine the concentration of proteins accurately.
Fast, easy, and reliable isolation of plasma EVs
Background proteins, cell debris, solutes, lipids, and other particulates are reliably removed by qEV Size Exclusion Columns, making it possible to isolate the EVs from plasma in just 15 minutes.
When fractions collected from a qEV Size Exclusion Column was analyzed, it was observed that plasma EVs eluted in fractions 7 to 9 (Figure 1) with a mono-disperse particle size distribution (Figure 2) with mode and mean particle diameters of 93 and 115 nm, respectively. EVs of the highest purity (2.19E9 particles/μg protein, Table 1) were present in fraction 7, while EVs of the highest concentration (3.5E10 particles/mL) were present in fraction 8.
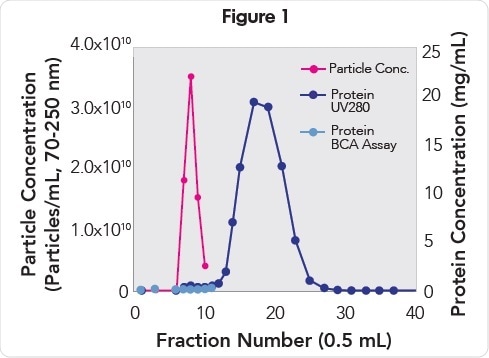
EV and protein concentration analysis of fractions collected from a qEV Size Exclusion Column. EVs (pink) elute in fractions 7–9 and are resolved from the proteins (purple and blue) that elute in fractions 11−30.
Table 1. EV and protein concentrations of fractions 7−9
| Fraction Number | Particle Concentration (particles/mL, 70−250 nm) | Protein Concentration (μg/mL)† | Concentration (particles/μg protein) |
|---|---|---|---|
| 7 | 1.8E10 | 8.2 | 2.19E9 |
| 8 | 3.5E10 | 28.0 | 1.25E9 |
| 9 | 1.5E10 | 36.0 | 0.41E9 |
† Total loading on the qEV Size Exclusion Column was 45 mg of protein.
The outcomes of the Pierce BCA analysis of fractions 7-9 revealed that 99.8% free from contaminating background proteins. Analysis of A280 protein also revealed that contaminating background proteins that eluted in fractions 11 to 30 were resolved from the fractions with EVs.
Anita Böing (Academic Medical Center, Amsterdam, NL), conducted a separate study, which showed that biomarker CD61 – a blood EV-derived marker – was abundantly present in fractions 7, 8, and 9 collected from a qEV Size Exclusion Column isolation of plasma EVs (Figure 3).
Conclusion
The fastest and easiest technique to isolate EVs from plasma is qEV Size Exclusion Columns. When compared to other techniques, qEV Size Exclusion Columns are free of contaminating proteins, HDL, and vesicle aggregates, and they are also gentle on the EVs which means their biological properties are not affected.
Moreover, additives are not used as these may interfere with downstream analyses, for example, RNA profiling, electron microscopy¸ ELISA, western blot, and proteomics. Fractions 7 to 9 are pooled for assays requiring the highest EV purity, and fractions 7 to 11 are pooled for assays requiring the highest EV yield.
qEV Size Exclusion Columns are easy, quality assured, and available off-the-shelf, allowing users to focus on their research rather than the isolation of their EVs.
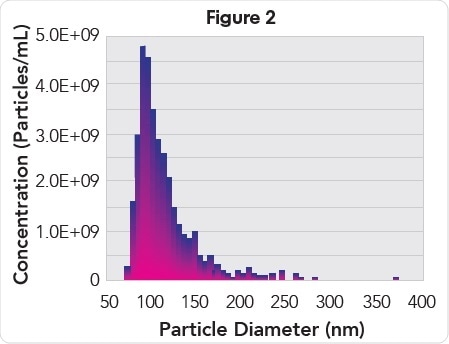
Particle size histogram of EVs from pooled fractions 7, 8, and 9.
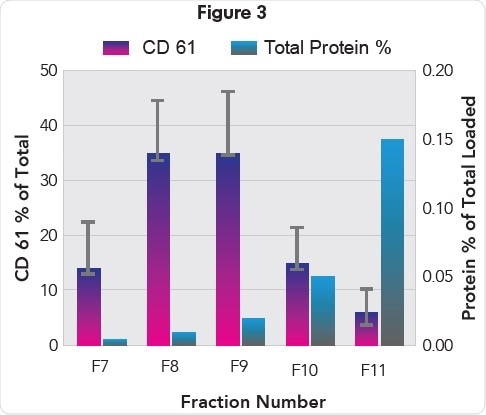
Protein (% of total) and CD61 levels in EV containing fractions 7-11 (Kindly provided by Anita Böing).
* If EV isolation is not performed on the same day as PFP preparation, store the aliquoted samples at -80°C until needed. Prior to isolating EVs, thaw PFP samples at room temperature and centrifuge (10,000 x g,10 mins) to remove any freeze-thaw aggregates.1.
1. Van Deun, J., et. al., “The impact of disparate isolation methods for extracellular vesicles on downstream RNA profiling,” Journal of Extracellular Vesicles, 2014;3:10.3402/jev.v3.24858. doi:10.3402/jev.v3.24858
2. Lobb, RJ, et al., “Optimized exosome isolation protocol for cell culture supernatant and human plasma”, Journal of Extracellular Vesicles, 2015;4:10.3402/jev.v4.27031. doi:10.3402/jev.v4.27031.
About Izon Sciences
 Izon Science designs and manufactures precision instrumentation for nano- and micro-scale particle analysis. The instrument systems, the qNano and qViro-X, are now in use in a wide range of research institutes and universities around the world.
Izon Science designs and manufactures precision instrumentation for nano- and micro-scale particle analysis. The instrument systems, the qNano and qViro-X, are now in use in a wide range of research institutes and universities around the world.The basis of Izon’s instrumentation is a unique, dynamically tunable nanopore technology platform which utilises a technology called Tunable Resistive Pulse Sensing (TRPS).
This technology platform is world-leading in offering an affordable, robust and highly flexible technology for real-time particle detection, quantitation and characterisation of individual particles at the nanoscale, across a wide range of applications.
TRPS represents a new paradigm in nanoparticle analysis, offering a unique alternative to the existing laser-based technologies available on the market. It offers applications not previously available to researchers and at a fraction of the cost of the incumbent technologies.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.
Last updated: Apr 19, 2018 at 10:59 AM






















.png)











No hay comentarios:
Publicar un comentario